ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Swati Yadav2, Arpita Sharma2, Guddi Choudhary2, Monika2, Alka Sharma1*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The inhibition potential of Azadirachita indica fruit extract on the corrosion of copper immersed in 0.5 N HCl at the room temperature (30°C) was investigated by the help of weight loss method. From the weight loss values, corrosion rates were determined. In the results obtained, the fruit extract, in hydrochloric acid medium, gave its best corrosion inhibition performance at 1.052 g/L extract concentration. The corrosion, kinetic and adsorptive parameters were calculated.
Keywords |
| Azadirachta indica, Acid corrosion, Kinetic Parameters, FT-IR Spectroscopy. |
INTRODUCTION |
| Copper has a wide range of applications due to its good properties. It is used in electronics, for production of wires, sheets, tubes, and also to form alloys. Copper is resistant towards the influence of atmosphere and many chemicals, however, it is known that in aggressive media it is susceptible to corrosion [1-3]. The use of corrosion inhibitors in such conditions is necessary since no protective passive layer can be expected. The use of inhibitors is one of the best methods of protecting metals against corrosion. Inhibitors which are in use are either synthesized from inorganic compounds and organic compounds having hetero atoms in their aromatic or long chain carbon system. These organic compounds can absorb on metal sheet and shows good anti-corrosive activity but these synthesized compounds are highly toxic and cause severe hazards to both human beings and the environment [4]. The safety and environmental issues of corrosion inhibitors arise in industries have always been a global concern. Since plants are biodegradable, readily available, non toxic and environmental friendly they are widely used as corrosion inhibitors for protection of metal in acid and alkaline environment [5-16]. Thus, recently researchers are focussing on natural product as corrosion inhibitor. The present study investigates the adsorption and inhibitive properties of ethanol extract of fruits of Azadirachta indica for the corrosion of copper in HCl solutions. Chemical constituents of Azadirachta indica fruit are mainly nimbin, nimbinin, nimbidin, metiantriol (triterpenoid), azadirone, azadiradionolide, meliacinin, limocin, epoxyazadiradione and the inhibitive effect is attributed to these phytochemicals present in the extract [17] - [25]. |
| Structures of the active constituents of Azadirachta indica fruit |
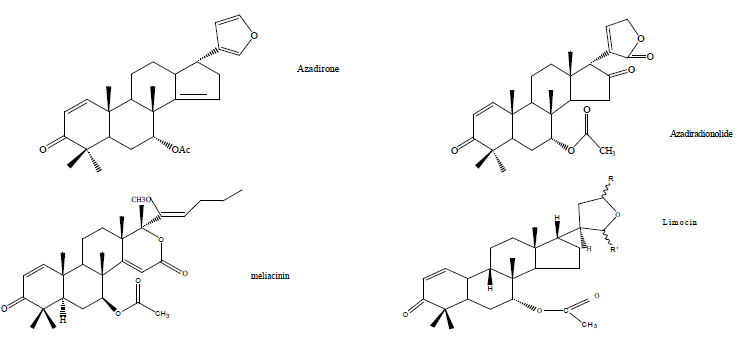 |
EXPERIMENTAL |
| Materials preparation / preparation of specimens |
| Copper sheet was mechanically pressed cut to form different coupons, each of dimension 3× 2.4× 0.16 cm with a hole of about 0.12 mm diameter near the upper edge for the purpose of hanging. Each coupon was degreased with acetone & double distilled water then dried in oven & thereafter preserved in a desiccator until cooled to room temp. |
| Composition of the metal was also analysed by carrying out the XRF studies by the help of Pay Analytical XRF instrument. |
 |
| Preparation of fruit extract |
| Plant material (fruits of Azadirachta indica) in natural condition was air dried (shade dried) for 20-25 days in shade. Then grained & powdered. 35 g of finely powdered dried material was taken in 1itre round bottom flask & sufficient quantity of ethyl alcohol (350 ml.) was added to cover the powder completely. The RBF was covered with stopper & left for 8-10 days. On the completion of soaking period, the ethanolic solution is refluxed by the help of soxhlet extractor for 24 hrs to concentrate the inhibiting chemicals. Thereafter, it is distilled & then filtered to remove any suspended impurities. The stock solution of the extract was stored in a clean corked bottle for further use. |
| Preparation of test solution |
| The solution of 0.5 N HCl was prepared using doubly distilled water. 50 ml. of the aggressive solution (0.5 N HCl) was measured in six separate 100 ml beakers labelled as C0, C1, C2, C3, C4, C5 & C6. EEAiF was added in the order of increasing concentration so as to have 0.0526, 0.1052, 0.2630, 0.5260, 0.7890 & 1.052g/L in C1, C2, C3, C4, C5 & C6 beakers respectively. No extract was added to the first beaker (C0). |
| Surface Morphology |
| (i) Scanning Electron Microscopy: |
| A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning it with a focused beam of electrons. The SEM of coupons of corroded (without and with the presence of the inhibitors) metal specimens was carried out by (ZEISS) instrument at 1000x magnification in the present study. The changes in the surface morphology of different coupons were analysed [26]. |
RESULT AND DISCUSSION |
| Chemical Method |
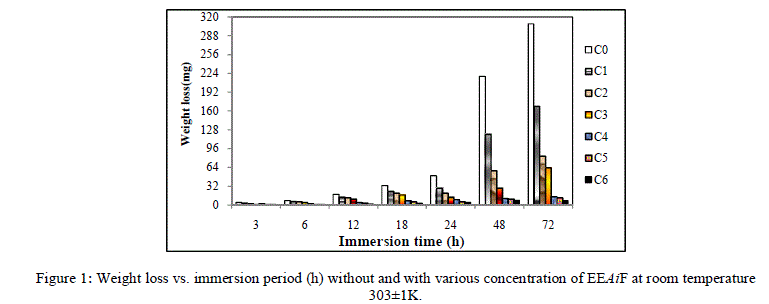
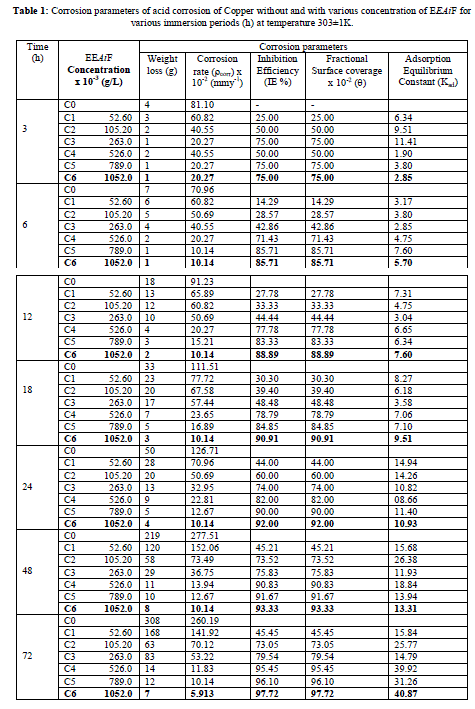
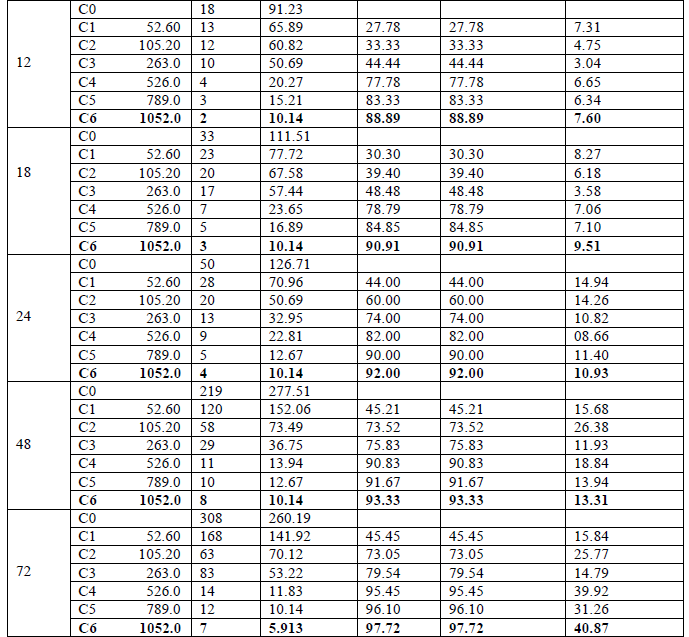
| The effect of inhibitor on the corrosion of Copper in HCl solutions was studied using the weight loss technique at room temperature at various immersion hours. Corrosion and adsorptive parameters like corrosion rate (ρcorr) in (mmpy), percentage inhibition efficiency (IE %), fractional surface coverage (Ãâè), adsorption equilibrium constant (Kad) etc. have been calculated and are tabulated in [Table 1]. |
 |
 |
| i. Effect of Concentration |
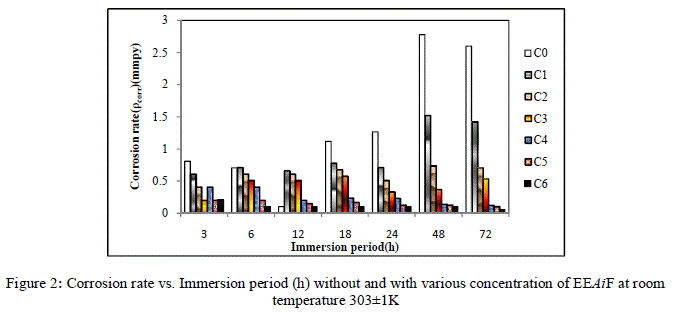
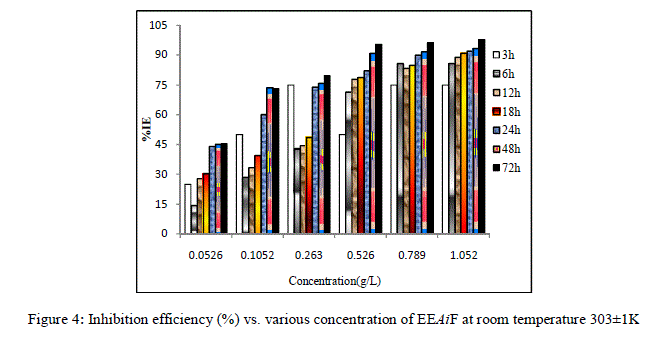
| From Figure [1] and [2] it is observed that the weight loss and corrosion rate both decreases with increase in concentration of inhibitor compared to blank. Inhibition efficiency of the inhibitor also increases with increase in inhibitor concentration. Maximum efficiency of 97.92% was achieved at 1.052 g/L at 72 hours. With the increase in concentration of the extract the surface coverage area (θ) on the metal increased hence the adsorption of the phytochemical constituents on the metal also increases forming a protective layer of inhibitor on the metal surface. This enhances the inhibition of the metal thus the corrosion rate decreases. |
| ii. Effect of Immersion Time |
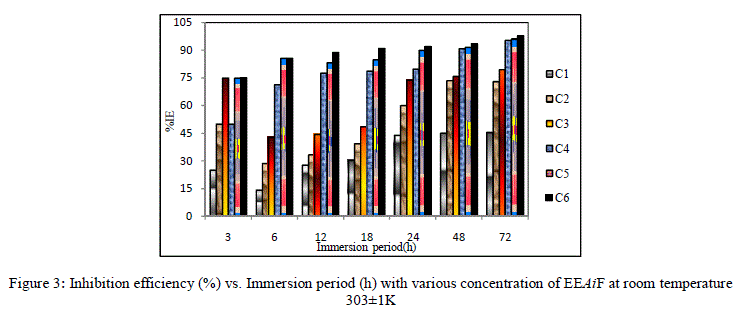
| For a given time of immersion, inhibition efficiency increased with the increase in concentration. At all concentrations, IE % increased with immersion time. A maximum efficiency of 97.92% was obtained at 72 hours. This increase of IE with respect to time of immersion indicates the stability of adsorbed layer on the metal surface. |
 |
| Figure 1: Weight loss vs. immersion period (h) without and with various concentration of EEAiF at room temperature 303±1K. |
 |
| Figure 2: Corrosion rate vs. Immersion period (h) without and with various concentration of EEAiF at room |
 |
| Figure 3: Inhibition efficiency (%) vs. Immersion period (h) with various concentration of EEAiF at room temperature 303±1K |
 |
| Figure 4: Inhibition efficiency (%) vs. various concentration of EEAiF at room temperature 303±1K |
| A. Kinetic treatment of weight loss results- |
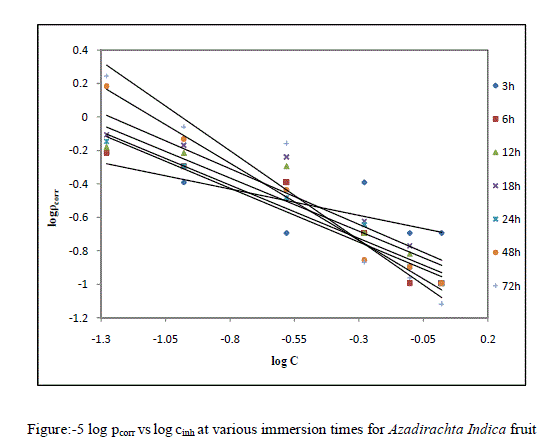
| The corrosion rate of Copper against the concentration of the EEAiF obeys the kinetic relationship as follow: log pcorr = log k + B log cinh ...(1) here k is the rate constant and equal to pcorr at inhibitor concentration of unity, B is the reaction constant which is the measure for the inhibitor effectiveness while C is the concentration of lines show that the kinetic parameters (k and B) could be calculated by Eq.1 and are tabulated in Table-2. Figure:-5 log pcorr vs log cinh at various immersion times for Azadirachta Indica fruit |
 |
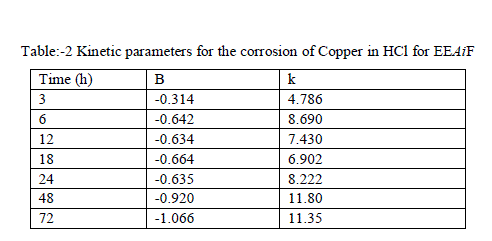 |
| The negative sign for the values of reaction constant B indicates that the rate of corrosion process is inversely proportional to the inhibitor concentration, i.e. inhibitor becomes more effective with increasing concentration hence when the change of pcorr with inhibitor concentration become steep (high negative value for constant B) it reflects good inhibitive properties for the EEAiF. |
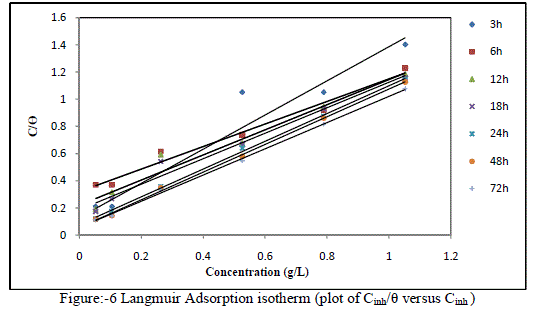
| B. Adsorption isotherm:- |
 |
| Corrosion inhibition occurred as a result of the adsorption of molecules of plant extracts onto the metal surfaces. It is known that the adsorption isotherms are very important for the understanding of the mechanism of corrosion inhibition. The most frequently used isotherms are Langmuir, Freundlich, Temkin and Frumkin adsorbtion isotherm. The action of inhibitor in acid solutions is generally agreed to be adsorption on to the metal surface which is usually oxide free in acid solutions. The adsorption of an inhibitor species on the metal surface interface can be expressed as a place exchanger process between the inhibitor molecules in the aqueous solutions and the water molecule on the metallic surface. |
| here x is the size ratio, representing the number of water molecules displaced by one molecule of inhibitor during adsorption process. When the equilibrium of the process reached it is possible to obtain different expressions of the adsorption isotherm plot, and thus the degree of surface coverage can be plotted as a function of the concentration of the inhibitor. The degree of surface coverage (θ) for various concentrations of the inhibitor has been calculated according to following equation. Langmuir isotherm was tested for its fit to the experimental data. Langmuir isotherm is given by |
| C/θ= 1/Kads+ C ............................ (3) |
| Where θ is the degree of surface coverage, C the molar inhibitor concentration in the bulk solution and Kads is the equilibrium constant of the process of adsorption. The plot of C/ θ versus C straight line graph were obtained (fig-6) which proves that Langmuir adsorption isotherm is obeyed for each temperature over the range of concentration studied. The correlation coefficient, slopes, obtained from Langmuir isotherm plots are shown in Table-3. |
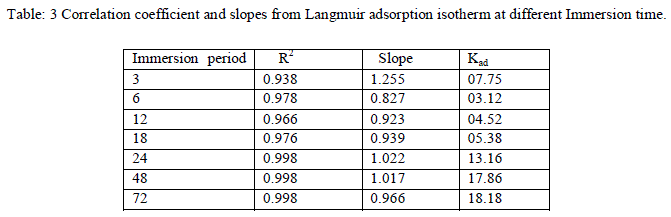 |
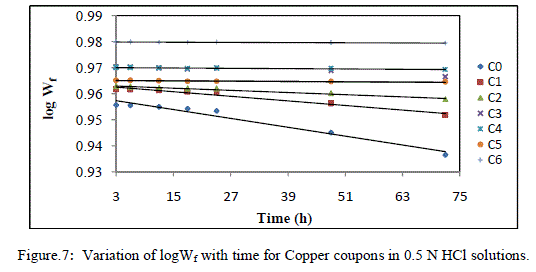
| The above data suggest that adsorption of EEAiF on the metal surface obeyed Langmuir adsorption isotherm with slope of almost unity, so monolayer of the inhibitor species must have been attached to Copper surface without lateral interaction between the adsorbed species. Corrosion reaction is an overall reaction in which both solid and liquid phase are consumed. It is therefore difficult to apply most of the principles of the chemical kinetics to corrosion reaction. In the present study of corrosion of Copper in HCl solutions, weight at time t (after post treatment of coupons) is designated Wf. When log Wf was plotted against time (h), a linear variation was observed, which confirms first-order reaction kinetics with respect to Copper in HCl solutions, formulated as: |
| log Wf = log Wo-Kt |
| Where Wo is the initial weight before immersion, k is the rate constant and t is time. Fig -7 depicts the plot of log of the weight of copper after treatment (log Wf) against time (t). |
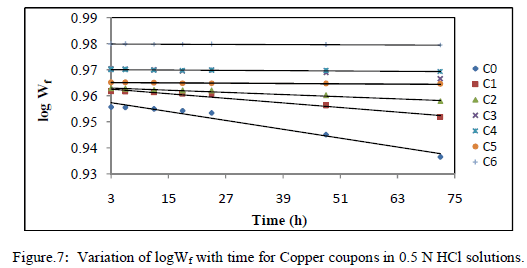 |
| C. Surface Morphology |
| Scanning Electron Microscopy |
| SEM studies (Fig. 10-11) shows that the inhibited metal surface is found to be smoother with fewer grooves as compared to uninhibited metal surface. As the inhibitor gets adsorbed by physiosorption and binds on the metal surface, it shows less abrasion as compared to uninhibited conditions. |
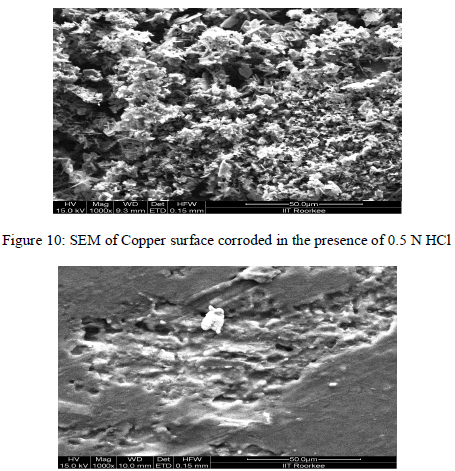 |
| Figure 11: SEM of copper immersed in 0.5 N HCl inhibitor containing 1.052 g/L resulting highest IE % (97.72). |
CONCLUSIONS |
| On the basis of present study the following conclusions can be drawn: |
| The corrosion rate decreases with increase in concentration of inhibitor with increase in immersion time. |
| The inhibition efficiency increases with increase of inhibitor concentration to attain a maximum value of 97.72% at 1.052 g/L. |
| The adsorption of extract molecule on the metal surface is spontaneous process which follow Langmuir adsorption isotherm. |
| Kinetic parameter clearly indicates the first order kinetics. |
| SEM analysis showed the presence of the compound in the plant extract react with the metal ion to form the layer of inhibitor on the metal surface. |
| The EEAiF acts as strong inhibitor for corrosion of copper in HCl medium. |
References |
|