ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
V Sreehari babu1, A. Jayanth kumar2 and S. Chandralingam3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Refractive indices and densities of binary mixtures like ethanol with benzene and methanol with benzene have been measured at room temperature (303K). From the values of the measured Refractive indices and densities, molar refractions (R), molar volumes (V), and electronic polarization (ε) of the mixed solvents were calculated. Intensity modulated fibre optic chemical sensor probes are designed and used to sense the binary mixtures at different concentrations or mole fractions
Keywords |
| Fibre optic chemical sensor, refractive index, molar refraction, electronic polarization |
INTRODUCTION |
| Prediction of refractive indices of binary liquid mixtures is essential for the determination of composition of binary liquid mixtures. Refractive index measurements in combination with density, boiling point, melting point and other analytical data are very useful industrially also for common substances which include oils, waxes, sugar syrups etc.[1]. Organic liquids are very important in various fields such as biomedical science, pharmaceuticals, solvent system for the refraction medium, chromatographic techniques and solventsystem in spectroscopy.Physical properties of a substance depend on the internal structure or the constitution of the molecule. Thus, the determination of properties such as refractive index, molar refraction, molar polarization, electronic polarization, etc. can provide valuable informations about the structure of molecules, binary, ternary mixture of liquids[2-5]. Fiber optic sensors (FOS) have received considerable attention in the recent years because of their inherent immunity to electromagnetic interference, safety in hazardous and explosive environment, high sensitivity and long distance remote measurements. The miniature size, low cost, intrinsic safety and ease of installation of FOS makes this system ideal for applications in various engineering areas including numerous in line chemical, food, beverages or medical analysis and monitoring system. L. M. Bali et al had developed an optical sensor for determining the proportional composition of two liquids in a mixture [6]. Some others developed fiber optical chemical sensors for refractive index, PH measurements, and organic pollutants in water[7-11] with various sensing mechanisms and different sensor designs. In the present work, an intensity modulated fiber optic chemical sensor for refractive indices, molar refractions and polarizations of binary liquids (Benzene with ethanol and benzene with methanol) was developed. |
MATERIALS & METHODS |
| All the chemicals used (Benzene, ethanol and methane) were of A. R. grade with purity >99%. The purity of liquids was checked by comparing experimental values of densities and refractive index of these liquids. The optical fiber used in the experiment was a standard commercial step index polymer optical fiber (POF) with a Polymethylmetacrilate (PMMA) core of diameter 0.980mm surrounded by a thin cladding layer of fluorinated polymer. The core refractive index (ncore) was 1.49 and the numerical aperture was 0.5. The refractive index of clad made of fluorinated polymer is 1.4036. Acetone is applied to the POF using a cotton bud and neutralized with the deionizedWater. Acetone reacted with the surface of the polymer to form milky white foam on the outer cladding which was thenremoved by the sand paper. The MFOE 71, 9508, Mexico, LED was used which provides the radiation at the wavelength of 630 nm. The length of the optical fibre used was 30 cm with a core diameter of 1mm, from which cladding of 6 cm was removed from the central region. The photo detector used was MFOD71, 9432, Mexico which converts the light intensity into the proportionate current. |
EXPERIMENT |
| Binary mixtures were prepared by mixing a known mass of each liquid in an airtight, stopper glass bottle. Refractive indices of pure liquids and binary mixtures were measured using thermostatically controlled Abbe’s refractometer with accuracy 0.001 units. Calibration was performed by measuring the refractive indices of doubly distilled water. The masses were recorded on digital balance Aux 220 series to an accuracy of ± 1×10-4 g. Care was taken to avoid contamination during mixing. |
| 25ml specific gravity bottle was used for density measurements. It was calibrated with freshly prepared triply distilled water. The density measurements for each experimental liquid were repeated three to four times so as to get low uncertainties of ±5x10-4gm/ml in density measurements. |
| From the measured values of densities and refractive indices, the values of molar volume (V), electronic polarization (ε), molar refraction were computed using the formulae: |
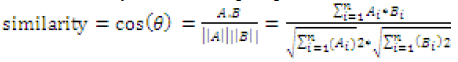 |
| Where, Rms is molar refraction of solution; n is Refractive index of solution; M1 and X1 are Molar mass and mole fraction respectively of firstcompound; M2 and X2 areMolarmass and mole fraction respectively of second compound and ρms is the density of binary mixture. |
 |
| The experimental set-up of the proposed electronic technique to determine the molar refractions of binary mixtures of different concentrations waisshown in Fig. 1. It consists of 5V constant dc supply connected to the LED. The light emitted by an LED is proportional to the forward current. Light emitting diodes are designed to produce coherent light at 630 nm wavelength with a very narrow bandwidth. |
| Silicon photodiode is being used as the photo detector in the circuit. The current of the photo detector is proportional to the intensity of the incident light falling on it. The photo detector is in the photovoltaic mode, which offers lower noise and therefore, is better suited for measurement and instrumentation applications. The Op-Amp’s input impedance being very high, the photo diode current flows through the feedback resistor. This current is converted into a proportional voltage given by |
RESULTS |
| The values of densities, refractive indices, molar volumes, electronic polarization and molar refractions for pure liquids were obtained and recorded in table 1 |
 |
| The values of densities, refractive indices, molar volumes, electronic polarization and molar refractions for binary mixture ofethanol and benzene at various mole fractions were obtained and recorded in table 2 |
 |
| The values of densities, refractive indices, molar volumes, electronic polarization and molar refractions for binary mixture ofmethanol and benzene at various mole fractions were obtained and recorded in table 3. |
 |
| The variation in the output voltage for each value of the concentration of benzene in ethanol was measured with the proposed electronic technique and was represented in Fig.4. The output voltage decreases almost linearly with increase in the percentage of benzene in ethanol-benzene binary mixture. As the volume of ethanol in the binary mixture approaches zero, the refractive index of the mixture approaches the core refractive index of the fiber and hence the output voltage drops to minimum value. |
 |
ACKNOWLEDGEMENTS |
| I sincerely thank my supervisor, Dr.A.Jayanth Kumar, professor of Physics, Institute of Aeronautical Engineering, Dundigal, Hyderabad, for his anytime support in my research work. I am very much thankful to Dr. S. A. Srinivas, Principal, Guru Nanak Institute of Pharmacy, Ibrahimpatnam, Hyderabad for allowing me to use their lab facilities as and when needed. The support given by my college management in doing the work is never forgettable, many thanks to them. I am also thankful to research scholars and Professor Sai Shankar for the guidance and help I received from them. |
CONCLUSIONS |
| Refractive indices, densities of binary mixtures were determined at various concentrations. From these measured values, molar volumes, electronic polarization and molar refractions at various concentrations of binary mixtures were computed. A plastic fibre optic sensor probe was designed to measure the variations in molar refractions of binary mixtures of benzene-ethanol and benzene-methane and was calibrated. It was observed that, the refractive index value and molar refraction increase with increase in Mole fraction of benzene. Also, the molar refraction value was observed to increase with rise of refractive index of the mixture. The output voltage decreases with increase of Molar refraction (or Benzene’s mole fraction) as is observed from the graphs in fig 4 and fig 5. |
| It could be seen that from table-2&3, the values of refractive index decreases with decreasein concentration of solution. As the concentration of solute decreases, distance between the molecules of soluteincreases. Hence refractive index, molar refraction and polarizability constant of ligand decreases. The substituentwhich has less effect of polarization has greater value of molar refraction and polarizability constant than othersubstituent.This data have been used to determine solute-solute, solute-solventand solvent-solvent interaction in the system. |
References |
|