e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, Faculty of Science, University of Colombo, Sri Lanka
Received date: 07/09/2015; Accepted date: 23/09/2015; Published date: 25/09/2015
Visit for more related articles at Research & Reviews: Journal of Chemistry
The number of multi drug resistant starins and strains with reduced suceptibility to antibiotics are continuoesly increased. Hence searching for noval antibiotics from medicinal plants are vital. An ethno medicinal survey conducted in two different districts in Sri Lanka, especially with tribal community showed the heavy used of Angiopteris evecta (Forst.) Hoffm to cure various types of diseases. The present study was carried out to examine the in vitro antimicrobial activity of A. evecta, a pteridophyte, against the bacterial strains of Escherichia coli (ATCC 35218), Staphylococcus aureus (ATCC 25928), Klebsiella pneumonia (ATCC 13883) and Streptococcus faecalis (ATCC 9790) by agar disc diffusion assay and the fungal stains of Aspegillus niger, Fusaium oxisforum and Curvularia sp by food poison technique. The crude extracts of A. evecta were prepared in dichloromethane, acetone, methanol, chloroform and ethanol from fronds, roots and rhizomes to select the best solvent to be used in the assay. Among all the extracts tested, acetone crude extract prepared from fronds, rhizomes and roots showed the highest activity against E. coli (ATCC 35218) and S. aureus (ATCC 25928). Compared to them, K. pneumonia (ATCC 13883) and S. faecalis (ATCC 9790) showed very low level of activity. Hence, the acetone extract was used to determine the minimum inhibitory concentration (MIC) of S. aureus (ATCC 25928) and E. coli (ATCC 35218) and the MIC values were determined as 290 μg/disc and 195 μg/disc respectively. The assessment of fungal toxicity was carried in terms of percentage of mycelial growth inhibition in five different concentrations (500, 400, 300, 200, and 100 μg/ml) of ethanol, methanol, dichloromethane, and acetone, for leaves, rhizomes and roots. The ethanol and methanol extracts of leaves, roots and rhizomes showed the highest activity against the all fungal strains tested. However, A. niger showed the highest activity showing 100% inhibition of the growth at 300 μg/ml of ethanol and methanol extracts. Taking together, our results showed A. evecta is a potential source to isolate novel antimicrobial compounds.
A. evecta, Antimicrobial activity, MIC, Food poison technique, Agar disc diffusion method.
Plants have been used as a source of medicines since ancient time. Most of the medicinal plants represent a rich source of antimicrobial agents. The value of medicinal plants to human livelihood is infinite. Therefore the screening of plant extracts for antimicrobial activity is beneficial and the active compounds may be useful in the preparation of improved herbal or drug formulations. In comparison to the higher plants, not much consideration has been given towards the pteridophytes in medicinal applications. However, the medicinal value of the pteridophytes are known to man for more than 2000 years and the tribal communities, ethnic groups and folklore in many countries have been utilized their parts like rhizomes, stems, fronds, roots and spores in various ways for the treatment of various ailments since ancient time [1]. Theophrastus (327-287 B.C.) and Dioscorides (100 A.D.) had referred medicinal attributions of certain ferns. The ancient medicinal works such as Sushruta and Charaka (100 A.D.) had mentioned medicinal uses of Marsilea munata linn and Adiantum capillus veneris Linn in their samhitas [2].
The pteridophyte flora of Sri Lanka which consists with 343 species distributed among 118 genera belonging to the 26 families including 58 species of endemic [3]. Sri Lankan pteridophyte flora has great scientific value as it has most primitive vascular plants namely Psilotum, Equisetum, Isoetes, Selaginella and Lycopodium [4]. However the investigation of their therapeutic prospective is vital as 13 pteridophytes species are already extinct from Sri Lanka and 90 species of fern and fern allies have been listed as threatened species [5].
As the indigenous knowledge proved a powerful and more effective strategy for the discovery of clinically useful compounds, an ethno medicinal survey was conducted to explore medicinally importance of pteridophyte flora used by local healers, local people and tribal people in two selected areas, Bulathsinhala in Kaluthara District and Mahiyanganaya in Badulla District in Sri Lanka. The Results revealed that, 27 species have ethno medicinal usage to cure 13 diseases and A. evecta is used to cure maximum number of diseases including wounds, constipation, hemorrhoids, dysentery, muscle pain, fever, and boils etc. [6]. A. evecta is a terrestrial fern belongs to family Maratiaceae and commonly called as giant fern or king fern. The present study was carried out to investigate the antimicrobial activity of A. evecta against the bacterial strains of E. coli (ATCC 35218), S. aureus (ATCC 25928), K. pneumonia (ATCC 13883) and S. faecalis (ATCC 9790) and the fungal stains of A. niger, F. oxisforum and Curvularia sp. According to our Results, A. evecta demonstrated the highest antibacterial activity against E. coli (ATCC 35218), S. aureus (ATCC 25928) and fungal activity against the A. niger.
Fresh plant parts; roots, rhizomes and fronds of A. evecta were washed 2-3 times with tap water followed by distilled water and the plant parts were cut into small pieces (5 mm) and shade dried at room temperature for a period of 2 weeks. An about 100 g of the samples were freeze dried and used for the solvent extraction.
Preparation of Crude ExtractFreeze dried samples of fronds, roots and rhizomes of A. evecta was mixed with chloroform, dichloromethane, acetone, ethanol and methanol in the ratio of 1:10 (w/v) separately for the crude extract preparation and they were kept in a sonicator for 45 minutes. Each mixture was filtered through the Whatman No: 1 filter paper and extraction procedure was done twice for complete extraction of the bioactive compounds. The obtained filtrates were combined together and concentrated at 35°C using rotary evaporator and the serial exhaustive extraction method was followed to obtain the fractions of each solvent [7]. The concentrated extract was completely dried by passing dry nitrogen and the weight of each residue was recorded. Each residue was dissolved in the respective solvent to prepare the concentration of 100 mg/ml stock solutions and they were kept in a refrigerator.
Test OrganismsThe bacterial and fungal strains selected for the present study were collected from Department of Plant sciences, University of Colombo, Sri Lanka. A total of four bacterial strains namely E. coli (ATCC 35218), S. aureus (ATCC 25928), K. pneumonia (ATCC 13883), S. faecalis (ATCC 9790) and three fungal strains, A. niger, F. oxisporum and a curvularia sp. were used for the present investigation. Identification and confirmation of strains, maintaining stock culture, quantification and growth of microorganisms were done according to the method described in Jayathissa et al. [8].
Antibacterial AssayThe bacterial cultures were maintained on nutrient agar slants at 4ºC and each of the microorganisms was reactivated prior to susceptibility testing by transferring them into a separate test tube containing nutrient broth and incubated overnight at 37ºC. They were suspended in saline solution 0.85% NaCl and adjusted to yield approximately 1.0 × 108–1.0 × 109 CFU/ml by using spectrophotometer at 600 nm. Antibacterial assay was carried out by disc diffusion method and the antibacterial activity of the test organisms were determined by measuring the diameter of zone of inhibition using venire caliper and expressed in millimeter [9]. The experiment was carried out three times and the Results were the mean of three replicates. Bacterial strains S. aureus (ATCC 9790) and E. coli (ATCC 35218) were used to determine the MIC value as these strains showed significant inhibition than others.
Minimum Inhibitory Concentration (MIC)Determination of MIC for plant extracts of A. evecta was carried out against the bacterial strains individually using standard disc diffusion method. The concentration series of test materials of 800, 600, 400, 200, 100 μg/disc were prepared by using stock solutions and the experiment was performed in three replicates for each concentration level and the antibacterial activity was expressed in terms of the mean of diameter of zone of inhibition (in mm) produced at the end of incubation period. Following incubation, the MIC values was calculated according to free diffusion and dissipative diffusion models as described by Bonev et al. [10].
Free Diffusion ModelFree diffusion model is based on the assumption that antibiotics diffuse freely in the solid nutrient medium 36 and the value of MIC is determined using a plot where squared size of the inhibition zone (x) plotted against the natural logarithm of the antibiotic concentration (c).
D is the diffusion coefficient, presumed to be independent of concentration, and the time of antibiotic diffusion.
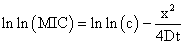
According to the dissipative diffusion model, value of MIC was determined using a plot where size of inhibition zone (x) plotted against the natural logarithm of the antibiotic concentration (c).
D is the diffusion coefficient, presumed to be independent of concentration, and V is a coefficient characterizing the dissipation rate. The choice of model can be made using the value of the regression coefficient R2 which is closer to 1
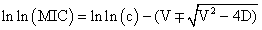
Fungal cultures were maintained on potato dextrose agar (PDA) slants at 4°C. A few single sclerotia of the fungus were obtained from the stock cultures of A. niger, F. oxisporum and curvularia sp and inoculated separately on the sterilized PDA plates. The plates were incubated at room temperature for 5-7 days to reactivate prior to susceptibility test [11]. Antifungal activity was determined by the poisoned food technique [11]. All crude extracts mentioned in antibacterial assay (ethanol, methanol, dichloromethane and acetone extracts of fronds, roots and rhizomes) were used in antifungal assay as well. Dried residues were prepared from each extract and dissolved in 2.5% dimethyl sulfoxide (DMSO) to prepare the stock solution with the concentration of 100 mg/ml. The rest of the concentration (100, 200, 300, 400, and 500 μg/ml) were prepared by mixing the appropriate amount of the stock solution with 10 ml of molten PDA 45°C and poured to sterilized plates (7 cm in diameter) [12]. The 7 mm circular fungi culture discs were taken from the margins of actively growing 7 days old culture of fungi by using sterile cork borer and kept in the center of the each fresh plate for the assay. Inoculated plates were incubated at room temperature for 4 days for A. niger and 7 days for F. oxisporum and Curvularia sp. The percentage inhibition of mycelia growth is calculated using the following equation by comparing the colony diameter of sample plate (with plant extract) and reference plate (with DMSO). The experiment was carried out three times and the Results were the mean of three replicates.
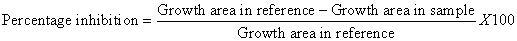
The information collected from an ethno medicinal survey conducted with tribal community revealed that different parts of A. evecta are being used to cure various types of diseases among them. Hence, antibacterial analysis of A. evecta was conducted with fronds, rhizomes and roots extract separately and crude extracts were prepared in dichloromethane, acetone, methanol, chloroform and ethanol to select the best solvent for the analysis. Each extract was tested against four bacterial strains of E. coli (ATCC 35218), S. aureus (ATCC 25928), K. pneumonia (ATCC 13883) and S. faecalis (ATCC 9790) and fungal stains of A. niger, F. oxisforum and Curvularia sp. The data collected from all experiments conducted were statistically analyzed and F test Results showed that there is a significant difference among plant parts tested and the different solvents tested on antibacterial activity at 95% confidence level. Except chloroform extract, all other extracts showed high activity against the S. aureus (gram positive) and E. coli (gram negative) and it showed low activity for ), K. pneumonia and S. faecalis. However, the highest activity was found with acetone extracts of rhizomes against the S. aureus 12.26 ± 0.32 mm (Figure 1a) and E. coli 10.05 ± 0.60 mm (Figure 1b). Similar to the statistical data Results, we could observe clear difference among the plant parts tested and rhizome extracted in acetone showed the highest activity resulting large inhibition zones (Table 1). Antibacterial analysis of A. evecta crude extract indicated that it could be a potential source to develop broad spectrum of antibiotics.
| Diameter of inhibition zone of acetone extract of 800 μg/disc (mm) | |||||
|---|---|---|---|---|---|
| Staphylococcus aureus | E. coli | ||||
| Fronds | Root | Rhizome | Fronds | Root | Rhizome |
| 8.09 ± 0.28 | 8.62 ± 0.32 | 12.26 ± 0.32 | 7.88 ± 0.60 | 10.05 ± 0.60 | |
Table 1. Zone of inhibition exhibited by S. aureus and E. Coli at 800 μg/disc acetone extract of Angiopteris evecta.
S. aureus and some strains of E. coli are human pathogen, which can cause number of diseases in human including endocarditis, osteomyelitis, toxic- shock syndrome, pneumonia, food poisoning, carbuncles etc. [13]. S. aureus is one of the bacterial strain that rapidly growing resistant towards antibiotics. More than 90% of S. aureus strains contain plasmids that encode beta lactamase, the enzyme that degrade many, but not all penicillin. Some strains are resistant to the beta lactamase resistant penicillin, such as methicillin and nafcillin and are evolved with methicillin resistant S. aureus (MRSA) and nafcillin resistant S. aureus (NRSA) [8]. Under this situation, uses of currently available antibiotics are uncertain and there is a need of searching for novel antibiotics active against them. Having strong activity of A. evecta against S. aureus and E. coli, further analysis was conducted with acetone extracts of rhizomes of A. evecta. The MIC assay was conducted for S. aureus (Figure 2a) and E. coli using disc diffusion assay. Interpretation of Results from disk diffusion method realizes on model dependent analysis [10]. The Results of sensitivity testing are important in choice of antibiotic and it is commonly reported as the MIC, which is defined as the lowest concentration of drug that inhibit the growth of organism. In most of the cases, assumptions of free diffusion of antibiotics may be incorrect and it leads to significant deviations of the predicted behavior from the experiment a of bacteria susceptibility to antibiotics [10]. Hence, the result of the disc diffusion method was analyzed using both free diffusion model and the dissipative diffusion model in order to find the MIC values for each strain. The model describes the agar diffusion assay that takes into consideration of loss of antibiotics during diffusion and provides higher accuracy of the MIC determined from the assay. The values are selected using the value of regression coefficient R2 which is closer to 1. According to the model, the Results showed MIC of S. aureus (Figure 2) and E. coli are 290 μg/ disc and 195 μg/disc respectively.
The antifungal activity of A. evecta was tested for A. niger, F. oxisforum and Curvularia sp by food poison technique. As described in antibacterial assay A. evecta, fronds, rhizomes and roots extracts were prepared in dichloromethane, acetone, methanol, chloroform and ethanol. Among the all extracts, ethanol and methanol extracts of fronds, roots and rhizomes clearly showed the significant antifungal activity against A. niger, F. oxisforum and Curvularia sp.
Curvularia sp, is one of the important members of family Dematiaceae (brown pigmented fungi), are a large and heterogeneous group are relatively common pathogens of animals and humans in both cases causing chronic nonspecific allergic sinusitis and traumatic skin infections [14]. Both ethanol and methanol extracts of fronds and rhizomes showed more than 60% inhibition of the Curvularia sp when the concentration was 300 μg/ml (Figures 3C and 4). However, 500 μg/ml of ethanol and methanol extract of fronds, roots and rhizomes were enough to cause 100% inhibition of Curvularia growth (Figures 3A and 4).
Total growth inhibition of F. oxisporum was observed with the ethanol extract of roots at 500 μg/ml concentration and 50% inhibition was observed at 200 μg/ml. However, ethanol extract of fronds and rhizomes showed 50% inhibition when the concentration was 300 μg/ml and 400 μg/ml respectively. These Results clearly showed the best antibacterial activity of F. oxisporum with Angiopteris evecta roots, when compared to fronds and rhizomes.
Among all the fungus A. niger showed the highest inhibition of mycelial growth and 100% inhibition was shown at 300 μg/ ml of both methanol and ethanol extracts of roots (Figure 5). Not only with methanol and ethanol extracts, A. niger could show 50% inhibition of growth with 500 μg/ml Dichloromethane and acetone roots extracts (Figure 5). A. niger is a human pathogenic fungus causing a group of diseases called aspergilloses. It produces carcinogenic mycotoxin in cereals, peanuts, chilli etc. and consuming of such food which contaminated with Aspergillus causes kidney and liver damages.
All the data collected for three fungal strains were subjected to the F test and the Results showed that there is no significant difference among plant parts to growth inhibition of the fungi and there is a significant difference among growth inhibition of fungi for the crude extracts prepared with different solvents at 95% confidence level. Similar to our observations, statistically analyzed data showed the response rate in methanol and ethanol extracts are higher than the dichloromethane and acetone extracts of leaves roots and rhizomes of Angiopteris evecta under 0.05 level of significance. It is indicated that significantly higher antifungal activity on A. niger than F. oxisforum and Curvularia sp. The methanol and ethanol crude extracts of leaves, roots and rhizome of A. evecta completely inhibited the A. niger growth at the concentration of 300 μg/ml. The significant antimicrobial activity of A. evecta revealed us that it is a potential source to isolate and purify of novel antimicrobial agents.
The acetone crude extracts of A. evecta roots, rhizomes and fronds showed antibacterial activity against S. aureus (ATCC 25928) and E. coli (ATCC 35218). However, the highest activity was shown by the acetone extracts prepared from rhizomes of A. evecta. The MIC assay Results revealed that the minimum concentration acetone, rhizome extracts required to inhibit S. aureus and E. coli are 290 μg/ disc and 195 μg/disc respectively.
The Results of the antifungal assay indicated that A. niger is the most susceptible fungus for ethanol and methanol extracts prepared from A. evecta roots showing 100% inhibition at 300 μg/ml. However, F. oxisforum and Curvularia sp also showed the 100% growth inhibition at 500 μg/ml indicating that A. evecta is a potential source to develop antifungal drugs.