e-ISSN: 2320-0812
e-ISSN: 2320-0812
Chandrakant Belwal*, Divyesh Patel, Kamlesh Chauhan, Yogendrasinh Parmar, Ajay Singh Rawat, and Anand Vardhan
Sterling Biotech Research Centre, A Corporate Research Centre Of Sterling Biotech Limited, Jambusar State Highway, Masar-391421, Vadodara, Gujarat, India.
Received date: 22/09/2014 Revised date: 28/09/2014 Accepted date: 30/09/2014
Visit for more related articles at Research & Reviews: Journal of Pharmaceutical Analysis
An unknown impurity in fermentation based drug substance lovastatin at 1.06 RRT was observed invariably in all batches when analyzed by HPLC as per Ph Eur. monograph. This impurity was isolated from the impurity enriched sample using reversed phase preparative HPLC and characterized by using spectroscopic (PMR, CMR, MASS and UV) and thermal analysis (DSC) techniques.
Lovastatin, Isolation, Characterization, Impurity, Chromatography
Statins are widely used to lower the blood cholesterol level in patients of hypercholesterolemia. They competitively inhibit the rate-limiting enzyme of cholesterol biosynthesis, 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase [1]. Among the statins, mevastatin was the first to be investigated as a fungal secondary metabolite, later followed by lovastatin (monacolin K) [2]. Lovastatin was the first statin approved by United States Food and Drug Administration as a hypocholesterolemic drug in August 1987 (FDA Orange Book Detail for application N019643 for approval for 20 mg tablets on Aug 31, 1987 and 40 mg tablets on Dec 14, 1988) [3]. Many microorganisms have been reported as lovastatin producing fungi [4,5].
Purity of drugs is an important factor for production of safe and effective pharmaceuticals. Most of the drugs obtained by fermentation process are purified either by solvent extraction procedure or by chromatography. Although solvent extraction procedures are used in most of the case for their ease and fast purification, chromatographic procedure has some advantage over solvent extraction procedure regarding purity of drugs [6].
During the routine analysis of lovastatin as per Ph. Eur., an impurity at 1.06 RRT was detected by HPLC method [7]. A comprehensive study has been done to isolate and characterize this impurity by spectroscopic techniques. The requirement of identification and characterization of the impurity in the final drug substances is extremely necessary to meet the stringent regulatory or customer requirements [8].
Materials
The chemicals and reagents used for isolation and analysis are as follows:
HPLC grade Acetonitrile, Supplier: Spectrochem, India
Ortho-Phosphoric acid, Supplier: S. D. Fine chem., India
Water: Highly pure water using Millipore Milli-Q plus purification system
Lovastatin, Source: Sterling Biotech Limited
High performance liquid chromatography (analytical)
A Waters Alliance separation module equipped with UV detector was used. Lichrospher RP Select- B column having dimensions 250 × 4.6 mm and 5 μm particle size was used for analysis. The column was maintained at 35 °C and the eluent was monitored at 238 nm and the data was recorded using Empower- II software. Mobile phase A (0.1 % phosphoric acid in water) and mobile phase B (acetonitrile) are used for the separation in an gradient system with a flow rate of 1.5 mL/min. The test solution was prepared by dissolving 20mg sample in 50mL of acetonitrile.
High performance liquid chromatography (preparative)
A Waters dataprep separation module equipped with 2487 UV detector and system controller was used. A combination of two columns namely Inertsil ODS-2 column having dimensions 250 × 50 mm, 10 μm particle size and Xbridge 30 mm × 50 mm, 5 μm particle size were used for the impurity isolation work. A 10 mL injection loop was used and the eluent was monitored at 238 nm and the data was recorded using Millenium software. About 1000 mg of the sample was dissolved in acetonitrile and loaded on preparative column. Mixture of water and acetonitrile was used as mobile phase. Flow rate was adjusted to 10 mL/min and the eluent was monitored at 238 nm.
Mass spectra were obtained using Waters Q-tof LC/MS-MS mass spectrometer in positive ion ionization mode.
Proton and carbon NMR measurement were performed on a Bruker Avance 500 MHz instrument at 25 °C in deuterated dimethylsulfoxide and chloroform and the chemical shift values were reported on the δ scale relative to TMS.
Detection of impurity
HPLC analysis of Lovastatin invariably exhibited an unknown impurity at RRT 1.06 when analyzed using method described in Ph Eur. Monograph (Fig. 1). The target impurity under study is eluting at retention time 10.69 min, and lovastatin is eluting at 10.09 min. Target impurity (at 1.06 RRT ) was isolated from enriched impurity sample of lovastatin on preparative HPLC.
Isolation of impurity by preparative HPLC
A reversed phase solvent system was used for the isolation of impurities. The enriched impurity sample was loaded on the preparative column and the pure fraction collected were combined together and analyzed using analytical HPLC to confirm the RRT and purity of the isolated impurity. The combined fraction was concentrated on Buchi rotavapour R-210 under high vacuum to distill out the solvent (acetonitrile). The remaining aqueous part was lyophilized using Vertex lyophilizer to get a pure compound. The chromatographic purity of the impurity is tested by analytical HPLC separately before and after concentration to check the integrity of isolated impurity (Fig. 2). The isolated solid impurity was subjected for spectral analysis.
Structure determination of impurity
Mass Spectroscopy
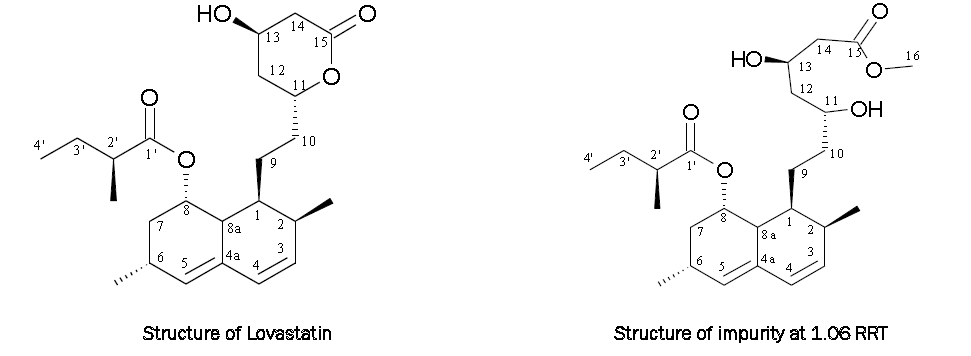
The mass spectrum of impurity at 1.06 RRT in positive ionization mode (Fig. 3) exhibits molecular ion peak at 437.24 [M+1] atomic mass units (amu) which correspond to the exact calculated molar mass of methyl ester of lovastatin acid form (436.58). Other fragment ions are at m/z 895.70 [2m+Na], 475.33 [M+K], 459.24 [M+Na], 422.36 [M-CH3], 405.33 [M-CH3-H2O], 317.26, 299.25 and 199.18.
1H-NMR Spectroscopy
Proton NMR measurement of isolated impurity were performed on a Bruker Avance 500 MHz instrument at 25 °C in DMSO-d6 and the chemical shift values were reported on the δ scale relative to TMS (Fig. 4).
1H-NMR (500MHz, DMSO–d6), δ 0.817-0.831 (m,6H), 0.997-1.040, (m,6H), 1.236-1.853 (m,11H), 2.237-2.503 (m,6H), 3.450 (m,1H), 3.573 (s,3H), 4.090, (m,1H), 4.470 (d,1H), 4.756, (d,1H), 5.190 (d,1H), 5.481 (s,1H), 5.777 (m,1H), 5.945 (d,1H).
Structure of isolated impurity was further confirmed by comparing 1H- NMR spectrum signals with those of lovastatin [9]. A sharp singlet appeared at 3.573 ppm (s, 3H, OCH3 (C-16)) which is not present in the 1H- NMR spectrum of lovastatin. It indicates the lactone ring of lovastatin is converted in to open acid form which undergoes esterification with methanol in presence of residual sulfuric acid.
13C-NMR Spectroscopy
Carbon NMR measurement of isolated impurity were performed on a Bruker Avance 500 MHz instrument at 25 °C in DMSO-d6 and the chemical shift values were reported on the δ scale relative to TMS (Fig. 6).
13C-NMR DEPT-135 Spectroscopy
Carbon DEPT-135 NMR measurement of isolated impurity were performed on a Bruker Avance 500 MHz instrument at 25 °C in DMSO-d6 and the chemical shift values were reported on the δ scale relative to TMS (Fig. 8).
Structure of isolated impurity was further confirmed by comparing 13C- NMR spectrum signals with those of lovastatin [9]. The –OCH3 signal appeared at 51.57 ppm (-OCH3 (C-16)) which is not present in the 1H- NMR spectrum of lovastatin, indicating the formation of mevinolinic acid methyl ester.
UV spectrum of impurity at 1.06 RRT and lovastatin were measured on a Perkin Elmer instrument in acetonitrile and the maxima was observed at 238.2 nm for both lovastatin and isolated impurity (Fig. 10).
Differential scanning calorimetry
The differential scanning calorimetry (DSC) curve for isolated impurity (1.05 RRT) was recorded in Perkin Elmer instrument at a heating rate of 10°C/ min under nitrogen atmosphere (Fig. 11). The thermogram is characterized by a single melting endotherm at 46.08°C with an onset temperature for melting of 39.26°C. The isolated impurity is a white waxy and low melting solid.
Possible route of formation of impurity
The impurity at 1.06 RRT was observed in all batches which were purified by charcolization and crystallization from methanol. The possible route of formation of impurity can be postulated in two step formation. In first step lovastatin is hydrolyzed to acid form of lovastatin (Mevinolinic acid) and second step include esterification of mevinolinic acid to form mevinolinic acid methyl ester.
Mass spectroscopy data of isolated impurity and lovastatin were compared and found that fragmentation pattern of both the compounds are same except the molecular ion peak or sodium adduct peak indicating the isolated impurity is structurally close to lovastatin. The UV spectrum of impurity is also indicating the impurity as related compound of lovastatin. 1H- NMR spectrum of isolated impurity suggest the presence of one –OCH3 group, presence of this groups can be confirmed by locating a sharp singlet of three protons at 3.573 ppm. 13C- NMR spectrum of isolated impurity also confirm the presence of one –OCH3 group, presence of this groups can be confirmed by locating a peak at 51.57 ppm (-OCH3 (C-16)) which is not present in the 1H- NMR spectrum of lovastatin, indicating the formation of methyl ester of lovastatin acid form. 13C- NMR DEPT-135 spectrum of impurity confirms the presence of four –CH2 in the molecule. The differential scanning calorimetry (DSC) curve for isolated impurity at a heating rate of 10°C/ min under nitrogen atmosphere shows a single melting endotherm at 46.08°C with an onset temperature for melting of 39.26°C.. Above spectroscopic data suggest that the unknown impurity at 1.06 RRT in lovastatin EP is methyl ester of lovastatin acid form.