ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ir. Renita Manurung, MT 1, Rakhmat Akbar Sinaga2 , Rahmad Taufik Simatupang3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Methyl esters have been widely used as the intermediate componds for a number of derivative products in oleochemical indutries, such as fatty alcohol, alkanolamide, monostearat glycerol and surfactant. The purpose of this research is to study the kinetics of amidation from methyl ester and diethanolamine by using sulfuric acid catalyst. The amidation, that is the reaction between methyl ester and diethanolamine was held in a glass batch reactor for 8 hours with mole ratio 1:1 using acid catalyst (H2SO4) 0,5 % (w/w) and 200 rpm stirring. The reaction temperature was varied from 120 to 160 0C, and sampling was performed every 30 minutes. The analysis includes Gas Chromatograpy-Mass Spectrometry (GC-MS) to determine the moleculer weight of methyl ester, analysis of ester value to know the number of amide that was formed, and Fourier Transfer Infra-Red (FT-IR) to know the structure of amide. The best result of the synthesis was obtained at temperature reaction of 160 0C (amide conversion of 98,36%). From the result of reaction was obtained that (-rA) = 0,02322 CA 0,1858 mol.dm-3.min-1 , k = 0,4069e(-2464,87/R)x1/Tand the activation energy was 2464,87 cal/mol.
Keywords |
| amidation, diethanolamine, diethanolamide, methyl ester, temperature, reaction rate. |
INTRODUCTION |
| Surfactant is an active compound which can degrade the surface tension (surface active agent) which also has a hydrophilic and hydrophobic group in a similiar molecule structure. These compounds can degrade the interfacial tension between two liquids phase that have a different polarity such as oil/water or water/oil. These unique properties make surfactant potentially used as an adhesif component, coagulant material, wetting, foaming, emulsifying, and material penetration and has been widely applied in various fields of industrial process that use the multiphase systems such as food industries, pharmacy, cosmetics, textile, polymers, paints, detergents, and agrochemical (Bailey, 2005). This dual properties makes surfactants can be absorbed at the air-water surface, oil-water, and solid-water, forming a single layer in which the hydrophilic groups are on the water and the hydrocarbon chains come into the air, contact with solid or submerged in the oil phase. Generally the nonpolar (lipophilic) is a long alkyl chain, while the polar (hydrophilic) is containing hydroxyl groups (Jatmika, 1998). |
| Nowadays, these surfactants are generally synthesized of petroleum derivatives and natural gases. In addition the raw materials sources that can’t be renewed, these surfactants which synthesized of petroleum and natural gases are also difficult to degrade by nature (Hilyati, et al, 2004). |
| Along with the increasing awareness of health and good environment, the demand of easily degraded surfactant (biodegradable) and plant based have also increased as well. So then, it’s required more studies to obtain a surfactant that has two criteria. They are derived from renewable raw materials, and degradative in nature, so it can be ecologically acceptable. One of the surfactant that contain both of these criteria are diethanolammide. |
| Alkanolamide is the result of reaction between primary or secondary alkanolamine with fatty acids, methyl esters, or triglycerides such as coconut oil. The composition and functions of alkanolamide can be fickle depending on the conditions and the type of reactants used. Alkanolamide as a nonionic surfactant can be applied for various uses. Fatty alkanolamide can be divided into four main groups, namely monoethanolamide (MEA), diethanolamide (DEA), monoisopropanolamide (MIPA), and ethoxylates or PEG alkanolamide. Each group has it own function and usefulness in a formulation (Swern, 1995). |
| Bernardini (1983) said that the alkanolamide can be produced in two ways, namely by reacting fatty acids with ethanolamine or by reacting esters with ethanolamine. Reacting fatty acids or methyl esters with ethanolamine will produce monoethanolamide. While reacting esters with diethanolamine will produce diethanolamide. Formation reaction of diethanolamide of the fatty acids compound and methyl esters can be seen in the following reaction : |
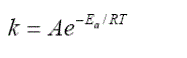 |
| The amidation is a formation reaction of an amide compound (Clason, 1986). According to Kirk and Othmer (1986), when the long chain fatty acids such as lauric acid and stearic acid combined with alkanolamine and heated at 140-160 0C, with or without a catalyst will result an amidation reaction. |
II. EXPERIMENTAL |
| 2.1 Materials and Equipments |
| The materials that used in this research are palm methyl esters, diethanolamine, sulfuric acid 98% as catalyst, ethanol, and KOH (pottasium hydroxide). While the equipments are a glass batch reactor, a stiring hot plate, condensor, and thermometer. |
| 2.2 Procedures of Amidation |
| The amidation, that is the reaction between methyl ester and diethanolamine was held in a glass batch reactor on the top of a stirring hot plate for 8 hours with mole ratio 1:1 using acid catalyst (H2SO4) 0,5 % (w/w) and 200 rpm stirring. The reaction temperature was varied from 120, 130, 140, 150 and 160 0C, and sampling was performed every 30 minutes.To observe whether the process was already underway optimal, the number of ester value was observed during this interval, in which the numbers are relevant to the amide formed. |
2.3 Analysis of samples |
| The analysis inculdes Gas Chromatograpy-Mass Spectrometry (GC-MS) to determine the moleculer weight of methyl ester, Fourier Transfer Infra-Red (FT-IR) to know the structure of amide, and analysis of ester value to know the number of amide that was formed. Analysis of ester value based on IUPAC in“Standard Method for the Analysis of Oil, Fats, and Derivatives.” |
III. RESULT AND DISCUSSION |
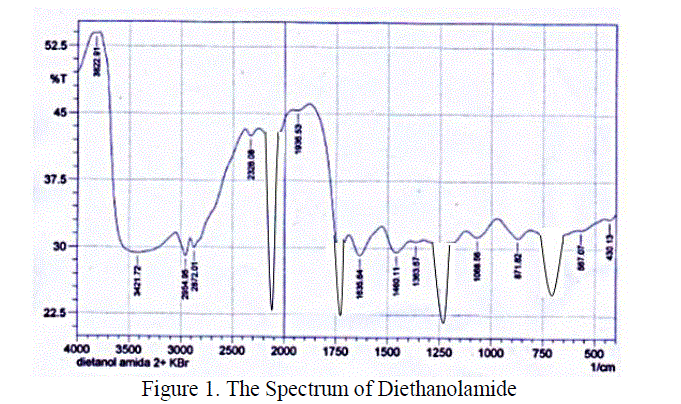
| Diethanolamide that synthezed has a group of molecules that can be identified by using FT-IR (Fourier Transform Infra-Red). Vibration peak at wave number 1732,08 cm-1 showed a stretching band C = O. While the O – H group is at wave number 2140,99 cm-1.The formation of diethanolamide is supported by FT-IR spectra which gives the absorption peaks at 1195,87 cm-1 which is the C – N stretching vibration. From the spectrum, it can be concluded that the diethanolamide has bees formed. Further analysis of the result by using GC-MS was obtained that the avarage moleculer weight of methyl esters was 277,04998 gr/mole. |
 |
| 3.1 Temperature Effect Against the Reduction of Ester Number |
| Quantitatively, the analysis of amidation was done by titrimetric method for determining the number of remaining esters. The number of ester value are relevant to the amide formed. Based on the temperature variation in the composition ratio 1:1 between methyl ester and diethanolamine and H2SO40,5 % (w/w) for 8 hours was obtained the following result: |
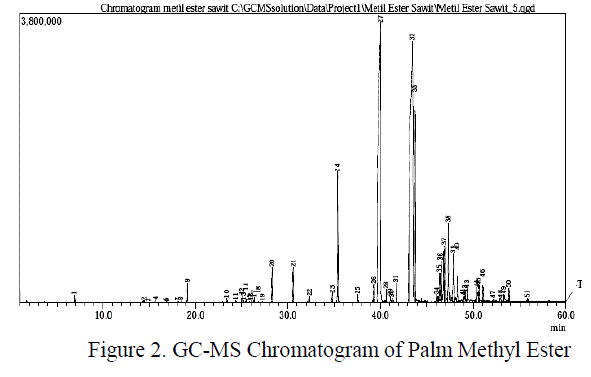 |
| Figure 3 showed that the reaction was influenced by temperature process. At relatively high temperatures the reaction will run perfectly. At low temperatures (120 and130 0C), the reduction of ester numbers were very slow and there were some fluctuations. These fluctuations indicate that the reaction was not stable or probably not a homogeneous reaction. Overall, the differences of ester numbers were not too flashy. Only on the time interval at the beginning of reaction showes a real difference.The conversion of amides was also showed an unsignificant diffrences. Especially at 150 and 160 0C, which gained 98.36 % conversion of amides. In Figure 3 was also observed some fluctuations at some points in each run. It was probably caused by the large vapour pressure itself inside the reactor and reduced contact between the reactants. |
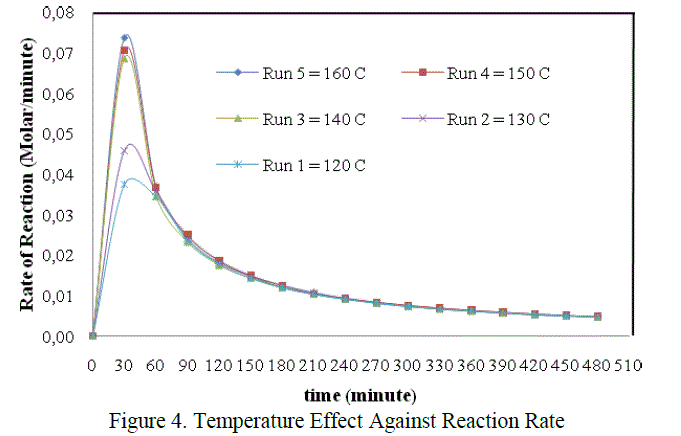
3.2 Temperature Effect Against Reaction Rate |
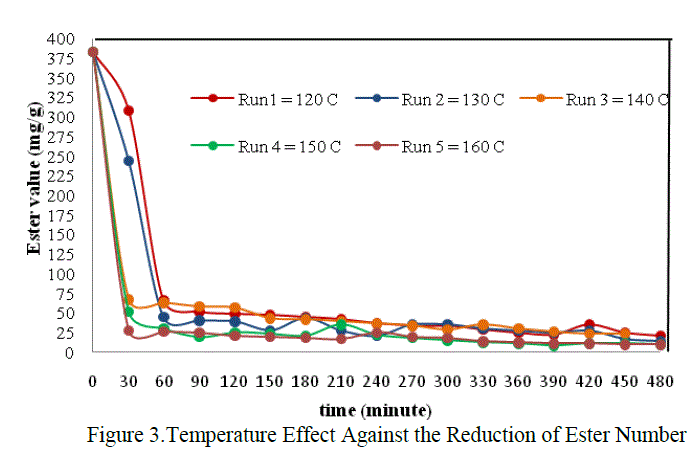 |
| The graph above showed that the effect of temperature on reaction rate, in which the reaction rate will be different on each temperature variation. But it was only seen at the beginnning of the reaction. From the figure above, the difference of reaction rate can be clearly seen at the time interval 0 – 60 minute only. The decreased of reaction rate at all temperature variations were tend to be the same after the 60th minute untill the end of reaction. In this case the most optimum of the reaction rate was found at temperature 160 0C, that is 0,0737 mol.dm-3.minute-1 when t = 30 minute. It was accordanced with the statement which stated that the chemical reaction rate increased in rising the temperature (Keenan, et.al., 1984) |
| The differences of reaction rate were very striking at the beginning of the reaction between T = 120oC, 130oC with T = 140oC, 150oC dan 160 oC. That was caused by the difference phase of the reactans. So that, the temperature in the beginning was very influential to activate the reactants and to help the appearance of the collision between reactants. The reaction was also helped by the agitation to accelerate the mass transfer and increas the collision between molecules. But as we get time the mixture will be more homogeneous and the effect of temperature and agitation were no more so influential on the reaction rate. It was suspected that the mixture was already homogeneous in the 60th minute. So at that time , the chemical reaction itself was the only factor which control the rate of reaction. Thus the decreased of reaction rate tend to equal in each run until the end of reaction. |
| The decreased of reaction rate in each run was caused by the reducement concentration of reactant as well as the time increasement that reacted to perform product or diethanolamide. It was accordanced with the equation (Levenspiel, 1999) : |
| (-rA) = -dCA/dt (1) |
| The equation above stated the amount of reactants that reduced per time unit. In which more time for the reaction will caused the concentration of reactants decreased. indeed the results obtained were relatively connected with the theory. |
3.3 Determination of Reaction Rate Constant k |
| From table 1 can be seen that the reaction rate constant (k) was increased in line as well as the increased of the temperature. But there was a little fluctuation at 140 0C in which the value of constant k decreased. It was suspected that the number of residual methy esters are bigger, since in this condition a small number of diethanolamine may have been formed the vapor phase that was not condensed perfectly. So that they can reacted with methyl esters. |
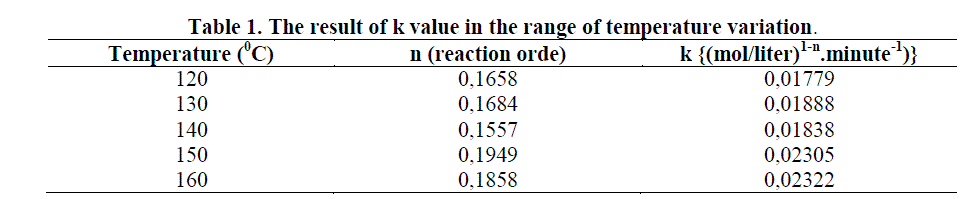 |
| Increasing the reaction temperature will accelerate the increasing of amide concentration, enlarged the decreased of methyl esters concentration (A), or increased the conversion of methyl esters (XA). It was caused of by increasing the reaction temperature, then the energy supply to activate the reactants and the collisions between the reactants to produce the reaction will also increase as well. From the results of research conducted, the reaction rate constant value (k) increased as well as the increasing of temperature. This was accordanced with the theory of Arrhenius and Westerterp’s statement (1984) who stated that increasing the temperature will also increasing the the reaction rate constant (k). |
| 3.4 Activation Energy |
| The value of k that has been obtained can be used to calculate the magnitude of activation energy by using the Arrhenius equation (Levespiel, 1999). |
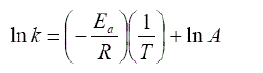 |
 |
 |
IV. CONCLUSION |
| a. The results of this research that conducted at the temperatur 160 0C was : the reaction orde (n) = 0,1858 ; the reaction rate constanta k = 0,02322mol 0,8142. ml -0,8142.minute-1 and (-rA) = 0,02322ïÃÆÃâ CA 0,1858 Molar minute-1 |
| b. The differences of reaction rate were very striking at the beginning of the reaction between T = 120oC, 130oC with T = 140oC, 150oC dan 160 oC. That was caused by the difference phase of the reactans. So that, the agitation and temperature in the beginning was very influential as energy supplier and mass transfer to activate the reactants and to reach the homogeneous. |
| c. The agitation and temperature were no more so influential on the reaction rate after the 60th minute since it was suspected that the mixture was already homogeneous. So that , the chemical reaction itself was the only factor which control the rate of reaction. |
| d. The increasing of temperature was effected against the increasing of reaction rate constant (k) at the time interval observed. |
References |
|