e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
Manish Gunjan1*, Jegathambigai Rameshwar Naidu1, Ishab Kumar2 and Yogesh Kumar2
Faculty of Medicine, Masterskill University College of Health Sciences (AMU), JB, Malaysia
Brahmanand Group of Institutions, Bulandshahr, UP, India
Received date: 21/01/2016 Accepted date: 01/02/2016 Published date: 06/02/2016
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
ABSTRACT Purpose: The purpose of the study was to find out the basic challenges and major aspects in launching of new pharmaceutical products, and the usual way to overcome with the concerned challenges. The second most important purpose was to find out the role of innovative and creative way in promotion of new pharmaceutical product and its impact on their market value. Methodology: I went through questionnaire based survey on 10 medical representatives and 10 doctors respectively to find out the challenges, strategies, innovations and considerations in launching of new pharmaceutical products. Later on I have analyzed the results by using statistical method and results were discussed accordingly. Findings: In this era of innovation and creativity, the “perfect product launch’ and lifecycle management is now viewed in a different and expanded way so the strategies would be accordingly, as the companies now a days think about virtually every aspect of research, marketing, product development, suppliers, materials management, manufacturing, distribution, warranty and defect management, maintenance repair and overhaul, and product end of life and disposal. So the challenges in launching and maintaining a new product in the market is increasing which is mostly being overcome through innovative and creative way while and after launching a product. Value: Innovation is global and without any boundaries. And hence its growth is being nurtured by active investments, grants, and tax incentive policies of established industrialized nations and emerging economies. This research might be helpful for that researcher having interest towards marketing and is also useful for the medical representatives whose company has recently launched a product or about to launch.
NPD, Management, Brand extension, Internal and external factors.
The new product launch phase is a critical part of the total new product development process. This is especially true in the consumer packaged goods arena, where nearly 26,000 new products were introduced in 1999 [1]. This compares to just over 12,000 new product introductions in 1986 [1]. With this dramatic escalation in the number of new products competing for consumer attention, the quality of launch programs greatly impacts the success of product introductions. In addition, each year it becomes more difficult to break through the noise generated by the thousands of existing products and line extensions. Sometimes, even well-conceived, innovative products meet with failure in the marketplace. Companies can end up pulling the plug on a perfectly good product because the launch strategy and execution failed to score high marks on the complex matrix of marketing factors that spell launch success.
Many companies adopt brand extension as strategy with the aim of benefiting from the brand knowledge achieved in the current markets. When a company launch new product and market under the umbrella a well-known brand name, failure rates and marketing costs are reduce [2]. Creating a brand name with well-established associations is one way of achieving this aim. Firms invest heavily in developing a brand. It is a very costly process but has many returns once success is achieved [3]. Brand extension as a marketing strategy has become even more attractive in today’s environment where developing a new product costs a lot of money and can be time consuming.
Launching of a new product is usually done through brand extensions. The newly introduced brand extension capitalizes on the equity of the already established (core) brand name [4,5] or even the company or corporate name. Consumer familiarity with the existing core brand name aids new product entry into the market place and helps the brand extension to capture new market segments quickly [6,7]. This strategy is often seen as beneficial because of the reduced new product introduction marketing research and advertising costs and the increased chance of success due to higher preference derived from the core brand equity. In addition, a brand extension can also produce possible reciprocal effects that enhance the equity of the parent brand [8]. Market is a place of competition and cost associated with introduction of new brand always soars, many firms are trying to decrease the risks involved in new product introduction and market the new product using the name of already well known existing brand as brand extension. Many firms use brand extension strategies to enter new categories. According to Ambler [9], it is common strategy of last decade that companies prefer brand extension rather than introducing a new product under new product name. Companies save their cost as well as minimize the risk by launching a new product as brand extension under the brand name of already wellknown brand. Brand extension strategy determines how far we can extend our brand. Before going into brand extension strategy we have to answer the following questions [10]
• Does the brand extension strategy consistent with the vision strategy?
• Does the brand extension strategy improve the brand image?
• Does the brand extension strategy consistent with the brand positioning strategy?
• What will be the impact on the brand in the case of unsuccessful brand extension?
Manufacturers of consumer products (CPs) are constantly working under tremendous pressure to generate revenue and improve operating efficiency [10,11] Challenges in meeting targets include changes in consumer’s demographics, competition in mature markets, expenditure on services, rise of private labels and the low success rate of new brands [12,13]. Today market is in an era of innovation and heavy competition. It is pervasive and influencing every industry or company. Today, companies think about virtually every aspect of research, marketing, product development, suppliers, materials management, manufacturing, distribution, warranty and defect management, maintenance repair and overhaul, and product end of- life and disposal [14,15]. Innovation is global and without any boundaries [16]. Its growth is being nurtured by active investments, grants, and tax incentive policies of established industrialized nations and emerging economies. In this era of innovation, the “perfect product launch’ and lifecycle management is now viewed in a different and expanded way [17,18].
The perfect product launch involves managing the development and support of complex products and services throughout the entire lifecycle from product design to product build to post-sale services [19]. It includes the integration of traditional new product with sourcing and procurement, supply chain planning and execution, and service-the entire product lifecycle management. The importance of being first to market is being discussed extensively [20,21]. Besides the instinctive idea that it is best to be first, other measurable benefits are shown in (Figure 1).
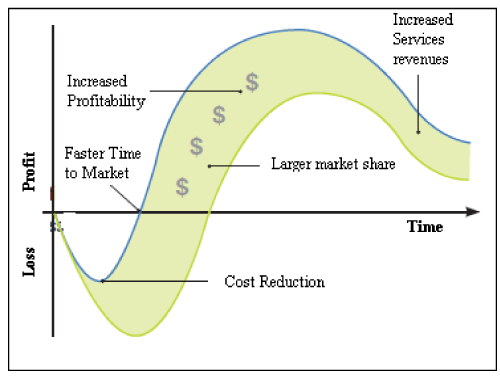
Figure 1: Market Sooner in PLM.
These benefits are for those who get to the market sooner with innovative products and services.
• Increased sales through longer sales life
• Increased margins [22]
• Increased product loyalty [23]
• More resale opportunities [24]
• Greater market responsiveness [25]
• Increased product loyalty [23]
Successful innovation has become a key driver for revenue growth, competitive margins and, in some cases, even for survival [26]. The ability to bring innovation to market quickly, efficiently and ahead of competitors is becoming increasingly important. An efficient product launch requires integration and coordination among multiple functional areas, including product design, procurement, planning, manufacturing process, sales and marketing [27]. In addition, as organizations increasingly leverage core capabilities of other companies, innovation has to be delivered through virtual networks, working with partners in a collaborative environment to bring product and services to market faster, smarter and cheaper [28]. Consequently, organizations now not only need to integrate internally, but also externally with suppliers and customers, creating end-to-end supply chain processes and capabilities which differentiate on product and customer requirements [29]. Improved learning capacity of organization is necessary to launch the product early in the market [30].
Successful innovation is of tremendous benefit to the organization while launching a new product. If the company can launch the product early in the market, it leads to the chance to acquire greater market share in introduction and growing phase of product life cycle management [31,32]. Product launching time is also very important for successful launch. Many parameters are important for successful product cannibalization [33]. Many parameters are important before launching the product. Customer psychologies, customer need, number of competitors in the market, price driven by the market are most critical and important parameters for successful product cannibalization. For successful product cannibalization study of product development phase and marketing phase is necessary [34,35] (Figure 2).
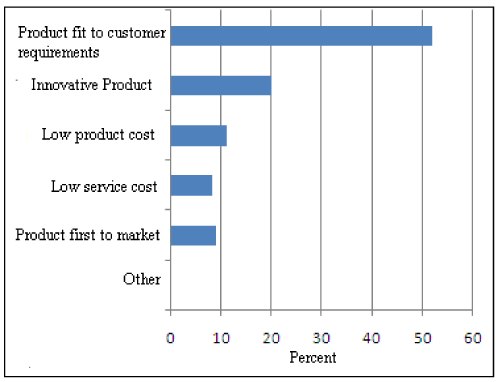
Figure 2: New Product Development.
Five parameters-Product best fit for customer, innovative product, low service cost, low product cost and product first to market are deciding factors in development of a new product. A study conducted in the case of a seasonal consumer product confirms these parameters. For launching a new seasonal product, product fit to customer requirement is the crucial factor. Near about 52% industries give importance to customer satisfaction and need as shown in Figure 2. In view of this, market research is a very important aspect to be conducted for perfect launch. For production of more and more innovative product, company has to concentrate on research and development sector. With increase in learning capacity of industry, innovative product comes out as per customers’ requirements. Study of Product Life Cycle reveals that customers’ viewpoints are the key factors for manufacturing a product fit for customer requirements [36] (Figure 3).
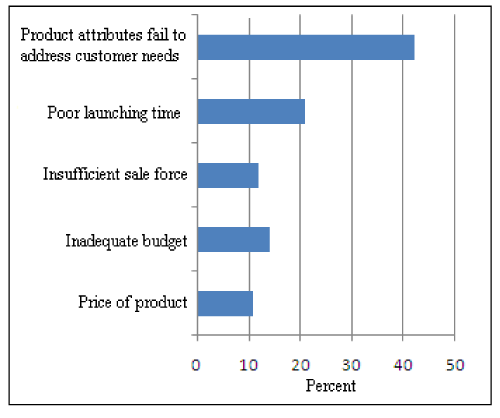
Figure 3: Causes of Unsuccessful Launching of Product.
Mainly five parameters are responsible for unsuccessful launching of a product (Figure 3). Here main cause of unsuccessful launching is that the product attributes fail to address the customer needs. Approximately 42% small scale consumer seasonal industries confirm this fact that the failure of a product is due to its attributes which fails to address the customer needs and ultimately results in unsuccessful launching of a product. 21% companies are of the opinion that poor launching time is the main cause of unsuccessful launching of a product. For successful launch companies has to concentrate on customer needs and right launching time.
For successful product launching mainly two parameters are vital as shown in (Figure 4). Product attributes as per customer needs and effective marketing strategies are the key factors for successful product launch. Nearly about 21% the company area of the opinion that scientific launching process is a vital parameter for successful product launch.
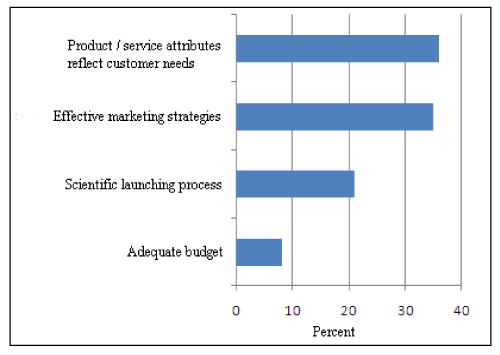
Figure 4: Key Strategies for Successful Product Launch.
The pharmaceutical industry is the world’s largest industry due to worldwide revenues of approximately US$2.8 trillion. Pharma industry has seemed major changes in the recent years that place new demands on payers, providers and manufacturers. Customers now demand the same choice and convenience from Pharma industry that they find in other segment. Indian Pharmaceutical Industry is poised for high consistent growth over the next few years, driven by a multitude of factors. Top Indian Companies like Ranbaxy, DRL CIPLA and Dabur have already established their presence. The pharmaceutical industry is a knowledge driven industry and is heavily dependent on Research and Development for new products and growth. However, basic research (discovering new molecules) is a time consuming and expensive process and is thus, dominated by large global multinationals. Indian companies have only recently entered the area. The Indian pharmaceutical industry came into existence in 1901, when Bengal Chemical and Pharmaceutical Company started its maiden operation in Calcutta. The next few decades saw the pharmaceutical industry moving through several phases, largely in accordance with government policies. Commencing with repackaging and preparation of formulations from imported bulk drugs, the Indian industry has moved on to become a net foreign exchange earner, and has been able to underline its presence in the global pharmaceutical arena as one of the top 35 drug producers worldwide. Currently, there are more than 2,400 registered pharmaceutical producers in India. There are 24,000 licensed pharmaceutical companies. Of the 465 bulk drugs used in India, approximately 425 are manufactured here. India has more drug-manufacturing facilities that have been approved by the U.S. Food and Drug Administration than any country other than the US. Indian generics companies supply 84% of the AIDS drugs that doctors without borders uses to treat 60,000 patients in more than 30 countries. However total pharmaceutical market is as follows (Table 1).
| Category | Value Market Share% |
|---|---|
| Anti-Infective | 16.4 |
| Gastrointestinal | 10.9 |
| Cardiac | 10.3 |
| Respiratory | 10.2 |
| Vit./Minerals/Nutrient | 9.6 |
| Pain/Analgesics | 9.5 |
| Dermatologic | 5.4 |
| Gynecology | 5.3 |
| Neuro psychiatry | 5.3 |
| Antidiabetics | 4.4 |
| Opthologicals | 1.7 |
| Others | 11.0 |
| Total | 100.00 |
Table 1: Total Pharmaceutical Market.
It is very much evident that chronic therapy area (Gastro, Cardiac, Respiratory, and Neuro Psychiatry and Ant diabetics) is dominating the market in long run.
While many pharmaceutical companies have successfully deployed a plethora of strategies to target the various customer types, recent business and customer trends are creating new challenges and opportunities for increasing profitability. In the pharmaceutical and healthcare industries, a complex web of decision-makers determines the nature of the transaction (prescription) for which direct customer of pharma industry (doctor) is responsible. Essentially, the end-user (patient) consumes a product and pays the cost. Use of medical representatives for marketing products to physicians and to exert some influence over others in the hierarchy of decision makers has been a time-tested tradition. Typically, sales force expense comprises an estimated 15 percent to 20 percent of annual product revenues, the largest line item on the balance sheet. Despite this other expense, the industry is still plagued with some very serious strategic and operational level issues. From organizational perspective the most prominent performance related issues are enlisted below:
• Increased competition and shortened window of opportunity.
• Low level of customer knowledge (Doctors, Retailers, Wholesalers).
• Poor customer acquisition, development and retention strategies.
• Varying customer perception.
• The number and the quality of medical representatives
• Very high territory development costs.
• High training and re-training costs of sales personnel.
• Very high attrition rate of the sales personnel.
• Busy doctors giving less time for sales calls.
• Poor territory knowledge in terms of business value at medical representative level.
• Unclear value of prescription from each doctor in the list of each sales person.
• Unknown value of revenue from each retailer in the territory
• Virtually no mechanism of sales forecasting from field sales level, leading to huge deviations
• Absence of analysis on the amount of time invested on profitable and not so profitable customers and lack of time-share planning towards developing customer base for future markets
• Manual and cumbersome administrative systems and processes designed which don't facilitate optimal efficiency levels in sales teams and many more.
Patents are a vital aspect of the global pharma industry. Patent protection is essential to spur basic R&D and make it commercially viable. But, only the developed nations endorse product patents. Most third world countries have patent laws but enforcement is totally lax. Some developing nations like India, Egypt and Argentina allow only process patent registration.
Prior to launching its products in any country, a pharma company undertakes patent registration to protect its own interests. To protect the interests of the consumers, it is necessary that the product be approved by the drug authorities in that country. Mostly the process for seeking approval is initiated alongside the patent registration process.
The level of competition in very high in acute segment on day to day basis however the degree of competition in not as much as high in chronic therapy area on day to day basis. As doctor has to prescribe drug for a long time in chronic cases and patient is supposed to consume it without any change of brand. While in acute cases doctor is changing brands on day to day basis.
• Super Core Model involving the search for and distribution of a small number of drugs from chronic therapy area that achieve substantial global sales. The success of this model depends on achieving large returns from a small number of drugs in order to pay for the high cost of the drug discovery and development process for a large number of patients. Total revenues are highly dependent on sales from a small number of drugs.
• Core model in which a larger number of drugs from acute therapy Area are marketed to big diversified markets. The advantage of this model is that its success is not dependent on sales of a small number of drugs (Table 2).
| YEAR 2010 | ESTIMATED |
|---|---|
| CVS | 18% |
| DIABETES | 3% |
| RESP | 7% |
| CNS | 8% |
| NSAIDS | 11% |
| NUTRA | 12% |
| GI | 13% |
| ANTI INF | 16% |
| OTHERS | 12% |
Table 2: Presents Market Share of Top Therapeutic Segment in the Year 2010.
However product choice will depend largely on the internal capabilities of the companies. Here it is very much evident from this projection that lots of opportunities lies with chronic therapy segment however growth is initially slow but it may generate good revenue in long run.
In pharmaceutical market there has been a significant shift from Acute towards Chronic Therapy area. Chronic segments are driving the growth of the market as leading prescribers in these segments are specialists as opposed to general practitioners. This is evident from high growth rates achieved by firms like Sun Pharma, Dr. Reddys laboratories and Dabur Pharma Ltd. Who have focused on these segments during last five years pharma companies have started identifying the hidden potential of oncological market. A number of drugs have been launched into the oncological market by pharmaceutical companies, including new biological drugs and drugs that can be used as a support for patients undergoing cytotoxic chemotherapy. As a result of the new, aggressive strategy, the aggressiveness of representatives has also been increasing, since the larger stress exerted by their companies might affect their stay in the company. Therefore, they tend to have more frequent visits to encourage doctors to prescribe drugs and thus increase sales. Pharmaceutical marketing is a specialized field where medical representatives form the backbone of entire marketing effort. Pharmaceutical companies also appoint medical representatives and assign them defined territories. Medical representatives meet doctors, chemists and stockiest as per company norms. Medical representatives try to influence prescription pattern of doctors in favor of their brands. The pharmaceutical distribution channel is indirect with usually three channel members i.e. depot/C&F, stockiest and chemist. For marketing of Pharma products companies require more and more skilled field force to develop good rapport with their direct customer (doctor). Moreover field force should have good product knowledge and USP of their products over other so as to convince doctors and Pull the demand for their products i.e. from Doctor to Retailer to Stockiest to CFA to company.
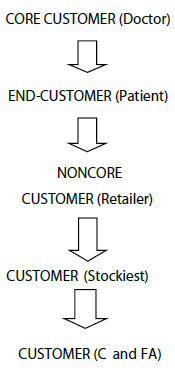
In this system, doctors are the core customers and the major thrust is given to build and retain these customer because they are pulling the demand for products hence companies also give main emphasis in building and retaining these customers. For retaining and developing customers, the companies normally provide gifts like sponsorship for various conferences like RSSDI, FOGSI, APICON, UPCON etc. For example Dabur having PASS (Professional Academic and Scientific Services) activities for promoting its chronic therapy range. Normally there are absolutely no chances of dumping of goods at stockiest and retailer level is yet reported also payment recovery of companies is also very good.
In present scenario companies are focusing more and more on the availability of products so as to enjoy good image in their customer’s (doctors) chamber. Many companies such as Glaxo, Pfizer, Dabur, FDC, Aventis, Cipla etc. are known for their availability of products. For marketing of these types of products field force should have good knowledge of product schemes and offers. Also field force is required to have a good rapport with retailers. Field force also required to ensure good availability of their products to convince doctors and push their products i.e. from to stockiest to retailer to doctor. It has been observed that sometimes there are more than fifteen or sixteen representatives in a day are meeting with their customer and requesting for same type of products. Although field force visits are important for an update on drugs and their use. The doctors are, in general, sneaking away, trying to hide from sales representatives, since there are too many and they are too pushy and there is too little time, and the representatives probably have noticed that the reluctant doctors have always less time for short meetings and less interest and tend to reduce the time of the visit.
CLOSING STOCK*2–OPENING STOCK=ORDER
The relationship between clinicians and representatives has always been good and pharmaceutical companies have provided, and still provide, the major economic support for customers' continuous medical education. Something needs to be done to find a solution to this problem that takes into account the needs of both pharmaceutical companies and their representatives on one side and physicians on the other, for a better professional interaction.
In this system, doctors and retailers are the core customers and the major thrust is given to build and retain these customers. Here retailers are also core customer as most of the times they are substituting the products based on their own discretion. For retaining and developing customers, the companies normally provide gifts like sponsorship for various conferences like small gifts and sponsorship to remind the products on daily basis.
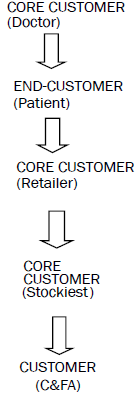
Also it is interesting to note that since this is a push system products are being pushed into the market so generally representatives place product orders from their stockiest on the basis of SKUs sold and schemes. Normally the chances of dumping of goods at stockiest and retailer level are reported also payment recovery of companies is also not very good. The focus is on efficiency and effectiveness and it includes fleet routing, deciding on timing and locations of delivery, scheduling and vehicle planning, etc [37,38].
A traditional Block Buster approach will still work for products targeting a large patient population and high perceived value and differentiation, but it will continue to require a focus on efficient launch activities (e.g., Merck’s diabetes drug Januvia, which emphasizes digital channels and patient education along with traditional feet-on-the-street and direct to consumer models). For products with a large patient population but perceived low differentiation by payers, pursuing a Value Buster approach could pay dividends, with a price point 20% to 30% lower to ensure access, and re-sourcing 30% to 50% below the traditional Block Buster (e.g., Sanofi’s colorectal product Zaltrap, which was forced to discount price 50% due to perceived similarity in outcomes to those of a competitor). For products with a smaller patient population but a high perceived differentiation, an Access Buster approach will make the most of price based on demonstrated value (e.g., Genentech’s highly successful Herceptin, which, while expensive, has retained its price because it demonstrated successful outcomes for HER2-positive types of breast cancer, with a companion diagnostic). The most challenging archetype, which we label a Turnaround Buster, includes products with a smaller patient population and low perceived differentiation. The best strategy for these products may be to refocus for other indications, delay the launch to capture additional trial outcomes or simply consider other options, such as out-licensing (e.g., Rare Disease Therapeutics’ Anascorp, a drug for scorpion bites with wide price variation that caused payer backlash) [39].
1. Literature Review
2. Preparation of questionnaire based on Literature review (for Medical representatives)
3. Preparation of questionnaire based on Literature review (for Doctors)
4. Distribution of questionnaire to ten medical representative (Randomly)
5. Distribution of questionnaire to ten Doctors (Randomly)
6. Analysis of results gathered from both medical representatives and doctors by statistical method.
7. Discussion on the basis of results
| Sr.No. | Product name | Company name | Year of launching | Current status(Monthly) |
| 1 | Vondervitinj | Adcock ingram | 2015 | 20,000 |
| 2 | Trimontemac | Macleods | 2015 | Not known |
| 3 | Abicef A.Z. | Bestochem | 2014 | 50,000 |
| 4 | Lovodil | Salveo life science Psychometric | 2013 | 50,000 |
| 5 | Calamal-D Kit | India Ltd. Indoco remedies Ltd. | 2014 | 30,000 |
| 6 | Durashape | Windlash Biotech Ltd. | 2014 | Not known |
| 7 | Max I.Q. | Ipca 3D | 2015 | 20,000 |
| 8 | Clothodone | Ravenbhel Pharma | 2010 | 2,83,333 |
| 9 | Haetaplox-cf | Voluenta Pharma Ltd. | 2015 | Not known |
| 10 | Raid plus | 2015 | 30,000 |
Table 3: Question 1: Which is your newly launched product?
I have gone through the survey on the basis of questionnaire, as the question no 1 is concerned, I asked about the newly launched product with 10 MRs from different companies, the result is mentioned in Table 1 along with the year of launching and current sell status accordingly. Out of 5 products launched in 2015 the best current status is for Raid plus from Voluenta Pharma Ltd. Company. Per month figure for the same is INR 30,000 (Table 3).
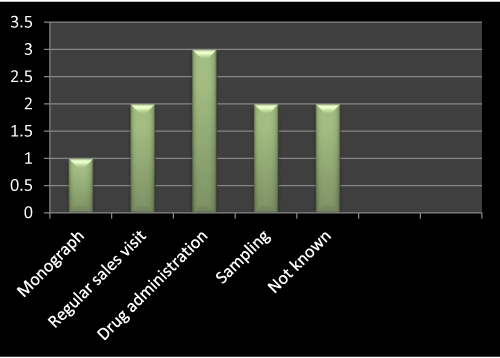
Figure 5: Question 2: How did you launch this product?
I have gone through the survey on the basis of questionnaire, as the question no 2 is concerned, I asked about the method of launching the product mentioned in Table 1 with the concerned MRs from different companies, as the result shows in Figure 5, out of 10, three has opted for drug administration, two has opted for regular sales visit, sampling and not known respectively. Only one has opted for monograph.
According to literature review I found that for launching of a new product drug administration based questions usually asked by the doctors and second most effective way of promotion is regular sales visit.
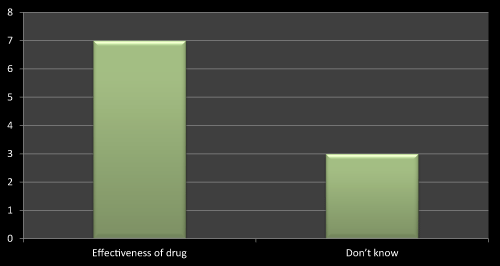
Figure 6: Question 3: What kinds of doubts doctor has while new product launch in the market?
On the basis of survey I found that mostly the doctors has doubt related to effectiveness of the newly launched product. As I went through the literature review I have found that most of the literatures shows that effectiveness of the drug is a vital point to be consider before launching and after launching a new product. So out of 10 MRs seven MRs replied that doctors has asked several questions related to efficacy of the drug while launching their products, three has no idea as they were from MNCs so they didn’t found or face any difficulties while launching a product (Figure 6).
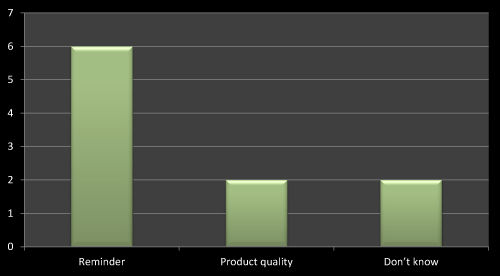
Figure 7: Question 4: How the name of product gets on the lips of Doctor?
On the basis of survey I found that the best way to promote a product is regular visit with reminder of newly launched product as this will help in remembering the product name on doctor’s lips. The same has been found in several literatures. The second important thing is product quality/efficacy which helps the doctors to remember the product name. Out of 10 MRs six has accepted that the best way to promote a product is reminder (Figure 7).
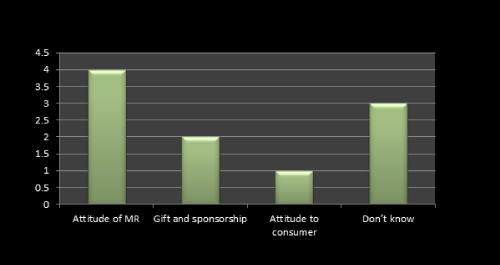
Figure 8: Question 5: What attitude do people have to promotion?
Attitude of MR plays a important role in launching a new product which we found even from the survey results. 4 MRs said that attitude of MR towards doctor, retailers etc plays a vital role in promotion of any product while gift and sponsorship is the next important point usually consider by the company. As per literature review we found the same (Figure 8).
As Figure 9 shows that attitude of MR influences the prescribing the new Product rather than gift and sponsorship the main point to be consider while launching a product is behavior and attitude of company representative consequently gift and sponsorship is another important thing to get prescription for new product. Attitude to consumer plays negligible effect on selling a new product since MRs are not direct in touch with consumer. As literature review shows that attitude of MR and gift and sponsorship is an important parameter in maintaining or launching new product in market (Figure 9).
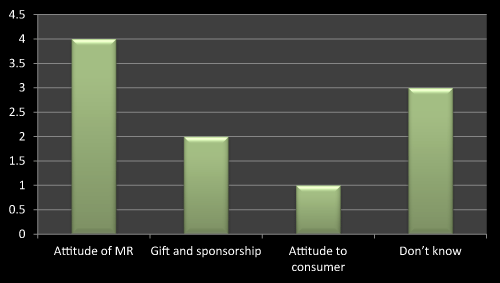
Figure 9: Question 6: Do you feel that accepting gift by doctor influences the prescribing the new product?
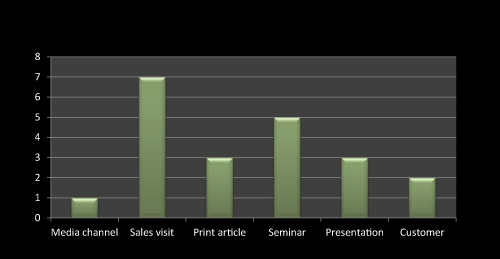
Figure 10: Question 7: What are the promotional activities you have done to promote this product?
As Figure 10 shows that sales visit and seminar enhances the sale of newly launched or existing product while print article presentation has comparatively minimal effect on pharma product sell. Customer and media channel plays negligible role especially in pharma product marketing. Most of the literature survey claims the same.
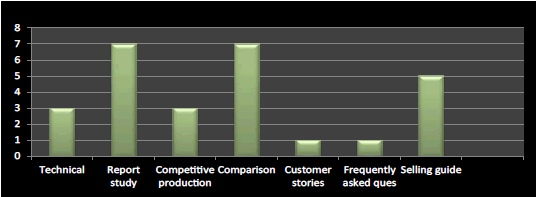
Figure 11: Question 8: What are the recommended tools for company’s product launch?
I have found from the survey that the important tool in product launch is report study, comparison and selling guide respectively and rest plays moderate to minimal role in product launch which has been verified and cross checked from literature (Figure 11).
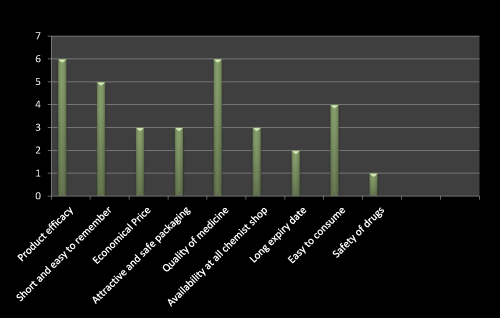
Figure 12: Question 1: Which one is the most important factor used to be consider while prescribing a new product?
I have gone through the questionnaire based survey on 10 doctors. Most of the doctor replied that product efficacy and quality of medicine is the first choice of consideration while detailing of newly launched product. Later on the short name which is easy to remember is more preferable. Only three doctors told that cheap, availability at all chemist shop, attractive and safe packed drug is their choice (Figure 12).
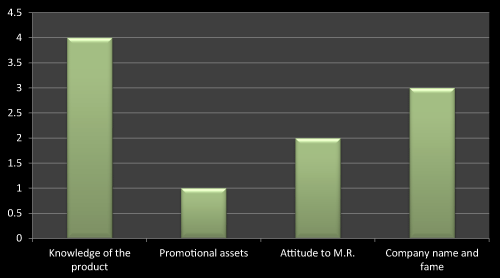
Figure 13: Question 2: What do you want to see in a Medical Representative while prescribing a new product?
For this question most of the doctors agreed that they keenly look into knowledge of the product and the company to which the concerned medical representatives belongs to, the reason behind this is, the company whose name is good in market that is because of their research based existing products, and so it’s easy to trust their brands. As literature reviews also favor to the same, the data obtained through questionnaire based survey is almost near to the literatures (Figure 13).
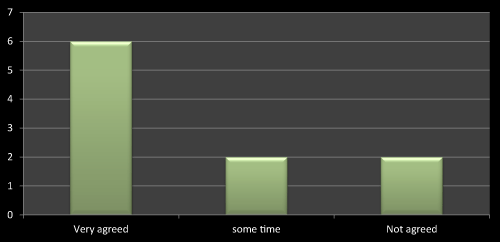
Figure 14: Question 3: Do you feel that discussion with sales representative affects the prescribing?
Discussions about the product with medical representatives is more important than anything as once the doubts clear it’s easy to prescribe the drugs according to concerned parameters. Another reason is its helps doctors to update their knowledge about new entity and their therapeutic effects. I got the same reply with most of the doctors and the same is stated in most of the literatures as well (Figure 14).
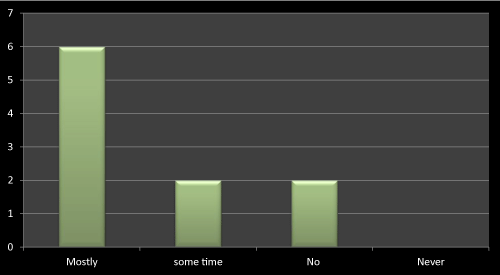
Figure 15: Question 4: Does company profile matters in prescribing any medicine?
Most of the doctors agreed that company profile matters a lot while prescribing a drug as the company whose name is good in market that is because of their research based existing products, and so its easy to trust their brands. So it’s easy for such companies to promote their newly launched products. As literature reviews also favor to the same, the data obtained through questionnaire based survey is almost near to the literatures (Figure 15).
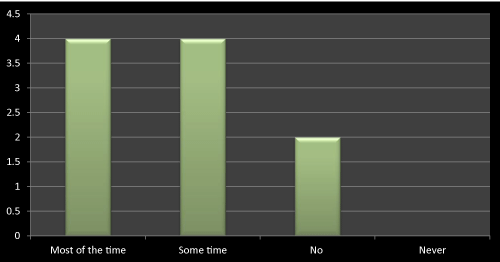
Figure 16: Question 5: Do you consider the research profile of a company while prescribing?
I got contradictory result for this question as out of 10 doctors 4 were considering research profile of the company while prescribing a medicine and on another hand 4 were not considering it all the time. It shows that there would be some other means of promotional way has to opt to promote the newly launched product to doctors. Innovative ideas may increase the sales of newly launched products, if doctors are not always look into companies’ research profile. I didn’t find the clear reason even in any literatures for these questions, as stated in some of the literatures that doctors mostly look into innovation and creativity while promotion of newly launched products as their expectations are same from all the companies and that is affordable and safe drugs for their patients (Figure 16).
Launching of a new product is usually done through brand extensions. The newly introduced brand extension capitalizes on the equity of the already established (core) brand name or even the company or corporate name. Consumer familiarity with the existing core brand name aids new product entry into the marketplace and helps the brand extension to capture new market segments quickly. This strategy is often seen as beneficial because of the reduced new product introduction marketing research and advertising costs and the increased chance of success due to higher preference derived from the core brand equity. In addition, a brand extension can also produce possible reciprocal effects that enhance the equity of the parent brand. Market is a place of competition and cost associated with introduction of new brand always soars, many firms are trying to decrease the risks involved in new product introduction and market the new product using the name of already well known existing brand as brand extension. Many firms use brand extension strategies to enter new categories. According to Ambler, it is common strategy of last decade that companies prefer brand extension rather than introducing a new product under new product name. Companies save their cost as well as minimize the risk by launching a new product as brand extension under the brand name of already well-known brand. Marketers believe that brand extensions are evaluated favorably by consumers because consumers transfer positive attitudes or affect toward the parent brand to its extension. The whole policy of the brand must be included for the brand extension strategy. Today, companies think about virtually every aspect of research, marketing, product development, suppliers, materials management, manufacturing, distribution, warranty and defect management, maintenance repair and overhaul, and product end of- life and disposal. Innovation is global and without any boundaries. And hence its growth is being nurtured by active investments, grants, and tax incentive policies of established industrialized nations and emerging economies. In this era of innovation, the “perfect product launch’ and lifecycle management is now viewed in a different and expanded way so the strategies would be accordingly.