ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| *A.C.Mongra Department of Biosciences, Himachal Pradesh University, Shimla Present address- Professor &Head Department of Biomedical Engineering &Biotechnology Adesh Institute of Engineering &Technology (Punjab Technical University) Faridkot, Punjab- 151203 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Thin layer chromatography of total lipids of Mastigocladus laminosus and comparison with values of standard indicated the presence of lipid spots, monogalactosyl diglyceride (MGDG), glycolipid (GL) and phosphatidyl diglyceride (PG) common in both high (45oC) and low temperature grown cells (26oC). Total saturated fatty acid content was five times higher in low temperature grown cells (25oC) compared to 45oC. Among important fatty acids detected in cells were caprylic acid (C8:0), nonanoic acid (C9:0), capric acid (C10:0), undecanoic acid (C11:0), lauric acid (C12:0), tridecanoic acid (C13:0), myristic acid(C14:0), pentadecanoic acid (C15:0), palmitic acid (C16:0), heptadecanoic acid (C17:0), stearic acid (C18:0), nondecanoic acid (C19:0), arachidic acid (C20:0), heneicosanoic acids (C21:0), behenic acid (C22:0), trichosanoic acid (C23:0), and lignoceric acid (C24:0). Low molecular weight saturated fatty acids species e.g. caprylic acid (C8:0) and nonanoic acid (C9:0), were totally absent in low temperature grown cells, while large molecular weight saturated fatty acid from carbon chain length C11 to C24. were abundant in low temperature grown cells. Higher amount of saturated fatty acid in cells grown at suboptimum temperature (25oC) indicated that membranes become highly rigid and as a result most of the membrane linked processes such as photosynthetic electron transport remains non-functional or less efficient at 25oC.
Keywords |
| TLC,HPLC,Mastigocladus laminosus,Lipid profile, thermophilic cyanobacteria,extreme environment |
INTRODUCTION |
| Lipids are esters of fatty acids and alcohols that comprise a large group of structurally distinct organic compounds including fats, waxes, phospholipids, glycolipids etc. Cyanobacteria may contain significant quantities of lipids (fats and oil) with compositions similar to those of vegetable oils. The lipids of some cyanobacterial species are also rich in essential fatty acids such as the C18 linoleic and y-linolenic acids and their C20 derivatives, eicosapentaenoic acids and arachidonic acid). These fatty acids are essential components of the diet of humans and animals and are becoming important feed additives in aquaculture (Borowitzka 1988). The lipids of cyanobacteria are generally esters of glycerol and fatty acids They may be either saturated or unsaturated. Some of the filamentous cyanobacteria tend to have large quantities (25 to 60 % of the total) of polyunsaturated fatty acids (Parker et al. 1967, Holton and Blecker 1972, Kenyon et al. 1972). A few cyanobacterial strains, which show facultative anoxygenic CO2 photoassimilation with sulphite as electron donor, lack polyunsaturated fatty acids in their lipids (Oren et al. 1985). Cyanobacteria contain four major glycerolipids:monogalactosyl diacylglycerol (MGDG), digalactosyl diacylglycerol (DGDG), sulfoquinovosyl diacylglycerol(SQDG), and phosphatidylglycerol (PG).MGDG represents 50-60% of the total glycerolipid content, and DGDG, SQDG, and PG each amount to 10-20%(Murata,1989).The cyanobacteria also contain minor amounts of monoglucosyl diacylglycerol (MGlDG), which is a precursor in MGDG synthesis. The fatty acids known to be present in cyanobacteria are palmitic acid (16:0), Palmitoleic acid can be abbreviated as 16:1Δ9 , hexadecadienoic acid (16:2 with unknown double bonds positions), stearic acid (18:0), oleic acid (18:1Δ9), linoleic acid (18:2Δ9,12), α-linolenic acid (18:3Δ9,12,15), γ-linolenic acid (18:3Δ6, 9,12), and octadecatetraenoic acid (18:4Δ6,9,12,15) (Murata, 1989 and Murata, et al 1992). Some cyanobacterial strains contain in addition myristoleic acid (14:1Δ9) and cisvaccenic acid (18:1Δ11). (Larisa etal 1999) The temperature-dependent changes in fatty acid composition were extensively studied in the mesophilic cyanobacteria Anabaena variabilis (Sato et al 1979 and Sato and Murata 1980), Synechocystis sp. PCC 6803 (Wada and Murata 1990) and Anacystis nidulans (Sato and Murata 1988 ) It was suggested that accelerated unsaturation of membrane lipids helps to maintain the membrane fuidity that is reduced under low temperature conditions(Murata and Wada 1995 ; Murata and Los 1997). Thus, fatty acid unsaturation is considered to be the key process in temperature acclimation in the mesophilic organisms.In present investigation quantitative changes in the fatty acid content at different temperatures fatty acid composition of the thermophilic cyanobacterium Mastigocladus laminosus were investigated in shifting of culture from optimum growth temperature to sub optimum temperatures .Induced changes in the the fatty acids in relation to fluidity of member has been studied |
II.MATERIAL & METHODS |
| II.1 Culture conditions The culture of Mastigocladus laminosus was isolated from hot water spring Tattapani(HP)48 KM from Shimla.The cells were cultured photoautotrophically at 45oC with continuous illumination at 100 μmol quanta m-2 s-1 and aeration with air that contained 2% CO2, in BG-11 medium (Stanier et al 1971) supplemented with 25 mM HEPES-NaOH, pH 7.5.. The cells were grown at 450C until the OD at 790 nm (OD790) reached 0.4 (about 3×107 cells ml-1).Then they were transferred to 450C,350C and 250C . Lipid extraction and fatty acid analysis were done from all the samples after 7 days of shifting of temperatures from initial growth temperature of 450C . All experiments were performed in duplicate II.2 Extraction and seperation of lipids The methods of extraction and sepration of lipids by thin layer chromatography used were of Nichols and Wood (1968) and Walsby and Nichols (1969), with a slight modification. II.3 Extraction Algal material was washed by centrifugation and extracted with chloroform: methanol, 2:3(v/v), clear extract was separated after centrifugation. The process was repeated until blue coloured residue remained. The extracts were pooled and concentrated by evaporating at 37oC. II.4Thin layer Chromatography Thin layer chromatography was carried out on the glass plates of size 20cm X 20cm size. Kieselgel Gnach Stahl silica gel of 5-25μ grain size containing 13% was mixed with distilled water in the ratio of 1.2(w/v) and layered on glass plates having 0.25cm thickness. Plates were activated at 120oC for 2 h After the application of extracts, the plates were developed in the chromatographic tank saturated with the solvent. The solvent system used was chloroform : methanol : glacial acetic acid : water, 85 : 15 : 10 : 3 (v/v). After development, the plates were removed and dried at room temperature. II.5 Visualization lipids II.5.1 Iodine method (Randerath,1964) Plates were exposed to iodine vapours in an air tight tank. All unsaturated and saturated lipids appeared as brownish yellow to yellow spots |
| II.5.2 Sulphuric acid method (Randerath, 1964) The plates were wetted with 25% sulphuric acid by dipping the lower end of the plates in the acid and dried at 150oC for 10min. Lipids appeared as charred black or pink spots depending upon their nature. II.6 Determination of Rf valves The plates were always run upto a known distance. The distance of each band migrated was measured and the Rf value was calculating in relation to the solvent front. II.7 Identification and quantification of fatty acids Identification and quantification of fatty acid was done by gas chromatography (Miller and Berger, 1985). using a Hewlett-Packard HP 5890 gas chromatograph equipped with a SP2330 capillary column. The fatty acid at three temperatures (25,35 and 45oC) was found out from the retention time (RT) and quantified them by using peak area and expressed as mg/g dry weight of sample. |
III. RESULTS & DISCUSSION |
| Lipid and fatty acid composition in diverse cyanobacteria have been studied by several workers (Wahal et al., 1973; Yadav, 1975; Rao and Talpsayai, 1982). Since cyanobacteria differ in their morphology and growth characteristics at different growth temperature, relative changes in the lipid spectrum are also expected. Results, from thin layer chromatography of total lipids in the present study and comparison with values of standards, indicated the presence of lipid spot, monogalactosyl diglyceride (MGDG) glycolipid (GL) and phosphatidyl glycerol (PG) common in both highand low temperature grown cells, when TLC plates were developed with H2SO4 acid treatment as shown in Fig. 1. |
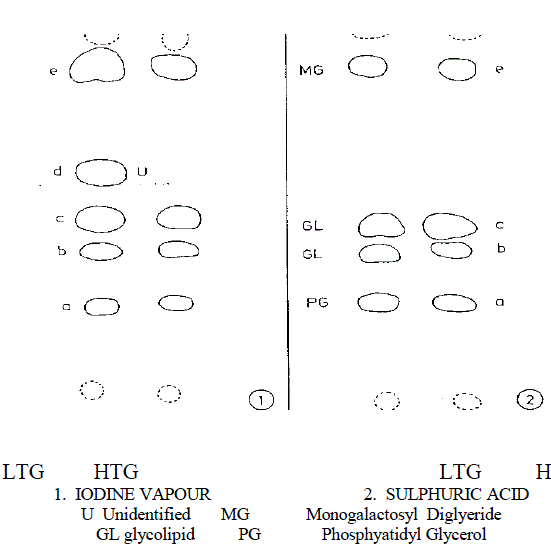 |
| Fig.1 Thin layer chromatogram (Diagrammatical) of lipids of the alga Mastigocladus laminosus showing effect of temperature on lipid components |
| Visualization 1 With iodine vapour a,c&e -Deep yellow b&d -Yellow 2 With sulphuric acid a- Light Brown d&e- Brown d- Dark Brown |
| One unidentified lipid spot was observed in low temperature adapted cells when TLC plate was developed with iodine vapours. This additional spot was absent in high temperature grown cells Fig 1. Nicholas (1973) reported the presence of glycolipid in cyanobacteria which includes MGDG, DGDG and SQDG. Except for some minor differences in the lipid spots found in LTG and HTG cells, no quantitative changes were observed. However amount of individual lipid species has not been quantified. Changes in both fatty acid species and their quantity were observed when the cyanobacterium was preadapted to different growth temperature for one week as shown in Table 1. |
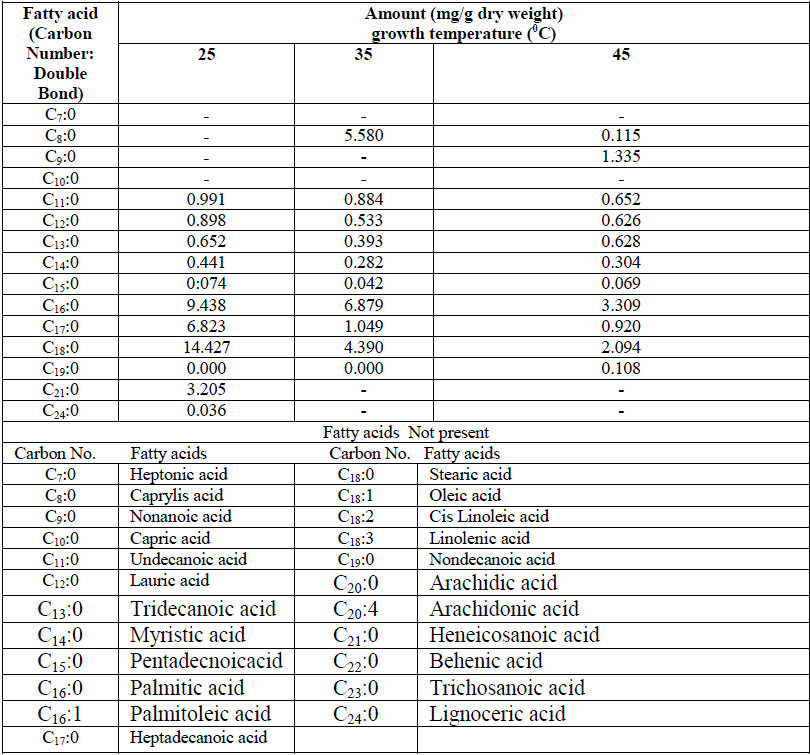 |
| Low molecular weight saturated fatty acid species e.g. caprylic acid (C8:0) and nonanoic acid were totally absent in low temperature grown cells, while large molecular weight saturated fatty acid from carbon chain length C11 to C14 were abundant in low temperature grown cells compared totheir high temperature grown counterpart. Heneicosanoic acid (C21:0) and lignoceric acid (C24:0) were totally absent at high temperature. Among predominant forms of saturated fatty acids, palmitic acid (C16:0,) heptadecanoic acid (C17:0) and stearic acid (C18:0) were significantly higher in 25oC grown cells and the trend of the above three species of saturated fatty acids progressively decreased with increase of growth temperature. Total amount of saturated fatty acids calculated from Table 1 at different growth temperatures have been depicted in the form of a histogram in Fig 2. A significantly higher saturated fatty acid content was in cells grown at 25oC as compared to 45oC. It is interesting to note that these variations in fatty acid profiles at different growth temperatures was a result of shifting of the culture originally grown at 45oC. |
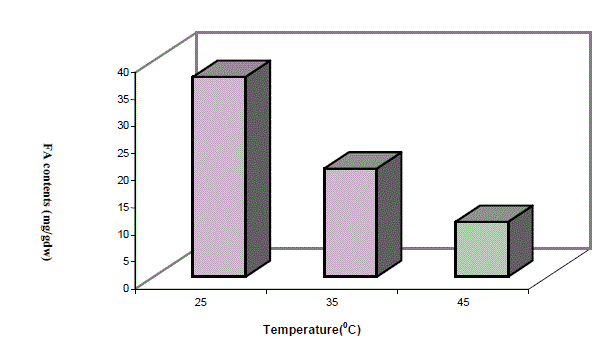 |
| Fig2. VARIATION IN THE TOTAL SATURATED FATTY ACIDS (FA) CONTENTS AT DIFFERENT GROWTH TEMPERATURES |
| In mesophilic cyanobacteria, the temperature-dependent changes in fatty acid composition were mainly assigned to MGDG, the neutral major galactolipid of the membranes (Sato,1979; Sato and Murata 1980; Sato, and Murata1988). It was also reported that in A. nidulans, which is able to produce only saturated and monounsaturated fatty acids, the decrease in temperature caused conversion of 16:0 to 16:1 in all lipid classes (Murata,1989). Analogous results were reported for lipid molecular species of the filamentous thermophilic cyanobacterium Mastigocladus laminosus (Hirayama and Kishida, 1991).In S. vulcanus, neutral galactolipids were not significantly affected by the temperature dependent desaturation. The changes in unsaturation pattern in S. vulcanus were caused mainly by desaturation of the charged lipids PG and SQDG ( Kiseleva etal .1999) The composition of fatty acids depends on the growth temperature.A change in faty acids and lipid after a shift of the growth temperature has been reported in Anabaena variabilis (Sato and Murata, 1980). A decrease in the saturated fatty acids at high temperature 45oC compared to 25oC does not reflect a conversion of saturated fatty acids to unsaturated ones as the later were not quantified. A reduction in the level of unsaturated fatty acids in S. lividus grown at high temperature as compared to low temperature grown cells has also been reported. Brock (1967a) reported that in the thermophilic M. laminosus, there is a total lack of polyunsaturated fatty acids. This low content of unsaturated fatty acids in the membrane of this thermophilic cyanobacterium may be a crucial factor for thermal adaptation. Higher saturation of fatty acids provides a stability to the membrane at higher temperature (David et.al., 1979). Due to the unique nature of M. laminosus i,e. a complete lack of polyunsaturated fatty acids, it is cosmopolitan and wide spread in almost all hot water springs in the world. The significance of higher saturated fatty acid content at lower temperature compared to higher growth temperature can not be explained. Miller et al. (1988) reported that the growth of a strain of Synechococcus species at 38oC caused an increase in the proportion of unsaturated fatty acids compared to its growth at 58oC. Unsaturated fatty acids have a lower melting point compared to their saturated analog and an increase in the degree of unsaturation causes greater fluidity of the membrane. The fatty acid compositions of cyanobacterial lipids show a considerable variation both when comparing different strains of the same genus and when comparing different growth conditions for a particular strain (Kenyon, 1972; Olsen and Ingram, 1975). A thermophilic strain of Synechococcus species contais no unsaturated fatty acid, while another species contains both mono and diunsaturated fatty acids (Kenyon, 1972). The proportion of mono unsaturated fatty acids in various thermophilic strains of Synechcococcus sp. when grown around 50oC varies from 25% (ForK et al., 1979) to 65% (Kenyon, 1972). Thus a general tendency towards less unsaturation and also a shorten carbon chain length is evident in thermophilic strains. The overall fatty acid composition is in general, a rather crude measure of the functionally important aspects of membrane composition. |
IV.CONCLUSION |
| In cyanobacterial cells, lipids are typically found only in the membranes Higher amount of saturated fatty acid in cells grown at suboptimum temperature (25oC) indicated that membranes become highly rigid and as a result most of the membrane linked processes such as photosynthetic electron transport remains non-functional or less efficient at 25oC. This type of study is useful to understand the function of plasma membrane at extreme temperature in shifting of temperatures. |
References |
|