ISSN: 2321-6204
ISSN: 2321-6204
1Ask Preparatory School, Nekemte Town, Ethiopia
2Department of Biology, Wollega University, Nekemte Campus, Ethiopia
Received date: 31/12/2018; Accepted date: 07/01/2019; Published date: 10/01/2019
Visit for more related articles at Research & Reviews: Journal of Food and Dairy Technology
These days, many people are eating away from home that could expose to foodborne diseases. The study was to test the quality and safety of some Ready-To-Eat (RTE) foods using the standardized microbiological parameters. A total of 75 samples comprising of each 15 ‘Beyeanet’, ‘Keyiwot’, ‘Pasta’, ‘Tibs’ and ‘Macaroni’ were collected from five cafeterias around Wollega University (WU) and analyzed for a load of different bacterial groups. Antimicrobial susceptibility tests were performed for the representative isolates using the Kirby-Bauer disk diffusion method. The mean (log) count of Aerobic Mesophilic Bacteria (AMB) was in the range of 6.041-6.441 with highest from ’Keyiwot’. Similarly, the highest mean counts (log) for bacterial endospores, Enterobacteriaceae, Total Coliforms (TCF), Fecal Coliforms (FCF) and Staphylococcus aureus was 4.923, 4.998, 3.893, 3.815 and 3.692 from ‘Beyeanet ’, ‘Tibs’, ‘Macaroni’, ‘Keyiwot’ and ‘Beyeanet ’, respectively. There was no significant difference in the level of contamination among cafeterias (p<0.05) with respect to AMB, Enterobacteriaceae, and TCF. A total of 107 bacterial isolates were collected from the food samples which were identified as 10 genera. The abundance of Bacillus>Staphylococcus>Alcaligenes>Acinetobacter>Pseudomonas>Aeromonas>Escherichia>Micrococcus>Plesiomonas>Citrobacter. However, no Salmonella and Shigella spp. were identified. Plesiomonas and Alcaligenes were resistant to gentamicin; Escherichia, Plesiomonas Citrobacter, and Alcaligenes were resistant to erythromycin; and Staphylococcus, Micrococcus and Acinetobacter were susceptible to ampicillin. The isolates were intolerant to ciprofloxacin; whereas 6 of the 10 genera were resistant to carbenicillin and oxacillin. In general, the bacterial load of RTE foods around WU was beyond the standard limit for consumption. Therefore, training for cafeteria owners and food handlers on how to handle RTE food can reduce the risk of contamination.
Antibiotics, Bacteria, Colony count, Dilution, Food items
Foodborne diseases are a major global problem causing considerable morbidity and mortality annually. According to WHO, [1] in developed countries, up to 30% of the population suffers from food-borne diseases each year, whereas in developing countries up to 2 million deaths are estimated per year. Gastro-intestinal diseases are of great concern to consumers, producers and policymakers in developing countries because it remains in the top five causes of sickness and death being contributed by unsafe foods [2]. According to the FDREMH [3], more than 250,000 children die every year from sanitation and hygiene-related diseases.
In recent years, eating habits have changed remarkably with many people eating away from home even though the food prepared and stored until consumption are susceptible to contamination [4]. Ready-to-eat (RTE) foods have been reported to be easily available, affordable, provide a diverse/variable food source, with a potential for improving food security and nutritional status [5]. However, according to Barrows et al. [6], RTE foods are considered high-risk foods because they do not necessitate any heating or processing prior to consumption. The source for contamination of RTE foods is contaminated raw food items, improper food storage, poor hygiene during food preparation, inadequate cooking and reheating of food items, and a prolonged time lapse between preparing and consuming the food items [7].
Different bacterial genera were reported from foods. A study conducted in different parts of Ethiopia showed the presence of pathogenic bacteria like Campylobacter, Salmonella, Staphylococcus aureus, Escherichia coli, Bacillus spp., Clostridium spp., Listeria spp. The viruses such as hepatitis and norovirus; and pathogenic molds and yeasts were also reported [8]. Study on a microbiological load of street vended foods from Addis Ababa [9,10] reported to contain pathogens above the minimum limit and unsafe for consumption.
Besides, a study on microbiological quality of milk in Adigrat showed that milk vending houses are higher in bacterial load than cafeterias [11]. Similarly, microbiological quality of RTE foods from cafeterias, restaurants, and juice vending houses in Mekelle City showed similar microbial load which is beyond the standard limit [6]. Even though studies of the microbial profile of RTE carried out in Ethiopia are relatively concentrated around Addis Ababa and cities like Bahir Dar, Gondar, Jimma, and Diredawa, the safety of these food items was not yet conducted on cafeterias in Nekemte town. Furthermore, research in the area of identifying the etiology food born disease is also at its infant stage in Ethiopia because of lack of well-developed laboratory system. Therefore, the study was aimed at assessing microbial quality and safety of RTE foods served in cafeterias around WU main Nekemte campus, Ethiopia.
Description of the Study Area
The study was conducted on cafeterias around WU main campus which is situated in Nekemte town, Ethiopia at 328 Km west of Addis Ababa. The site was purposively selected because it is the most populous area in the town consisting of students, staff, and daily laborers; and most of them consume RTE from the cafeterias around the university.
Study Design and Sample Collection
A cross-sectional study was carried out to assess the microbial load of RTE foods viz “Beyeanet”, “Keyiwot”, “Pasta”, “Tibs” and “Macaroni” that were served in twenty cafeterias (considered as total population) around the University. Five cafeterias were considered as representative of the population for the study. The samples were collected randomly from the five randomly coded cafeterias (A, B, C, D, and E). The five food items were sampled in triplicate from the five cafeterias during the June 2017 to March 2018.
Sample Preparation and Culturing
Wholemeal was purchased during the lunchtime and collected in sterile cans after which they were immediately taken to the laboratory, WU department biology for assessment. Then twenty-five grams of each food item was weighed on the electronic balance and added into multi-functional food blender (Model 7SBLG-371 Germany) into which 225 ml of 0.1% buffered peptone water was added gradually while it was being homogenized for about 2 minutes using aseptic techniques. From each homogenate, 1 ml was added to 9 ml of sterile 0.1% peptone water in test tubes and diluted serially to obtain dilutions up to 10-4 [12]. The dilute from 10-2 -10-4 were spread plated on pre-solidified solid media such as Nutrient agar, MacConkey agar, Xylose lysine deoxycholate agar, Eosin methylene blue agar and Mannitol salt agar for microbial counts.
Enumeration of Different Bacterial Groups
Inoculants (0.1 ml) for the enumeration of bacterial groups were taken from 10-3 and 10-4 dilutions of the samples and spread plated in duplicate on agar media. The number of bacterial groups on the original sample was estimated by the formula
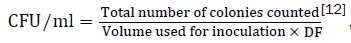 DF=dilution factor of the sample
DF=dilution factor of the sample
The culture media used for the enumeration of AMB was nutrient agar and the incubation temperature was 35℃ for 48 hrs. To estimate the load of Bacillus, the culture was kept in a water bath at 80℃ for 12 minutes, inoculated on nutrient agar, and incubated at 35℃ for 48 hrs [13]. Count of Enterobacteriaceae and Coliforms count was done on MacConkey agar base to which 1% of D-glucose was added and incubated at 35℃ for 24 hrs. Pink to red-purple colonies were counted as members of Enterobacteriaceae that ferment glucose but not lactose [9]. The number of fecal coliforms was estimated on Eosin methylene blue agar plates and incubated at 45.5℃ for 24 hrs. Then colonies with a metallic green sheen and/or purple-black were counted as members of fecal Coliforms. Enumeration of Staphylococcus aureus was done on Mannitol salt agar. The plates then incubated at 35℃ for 48 hrs after which golden-yellow colonies or mannitol fermenters (colonies that convert the medium from red to yellow) were counted as suspect colonies of Staphylococcus aureus and finally confirmed by coagulase test [9].
Isolation and Identification of Salmonella and Shigella Spp.
The original homogenate culture was streaked on plates of MacConkey agar, Salmonella-Shigella (SS) Agar, and Xylose Lysine Deoxycholate Agar. The colonies were picked and inoculated on triple sugar iron agar (TSI) for detection of fermentation of sugars, H2S production, and gas production; on lysine iron agar (LIA) for lysine decarboxylase/lysine deaminase test; on Simmon’s citrate agar for citrate test; on urea agar for urease test; and on Sulfide Indole agar for motility and indole production test [14].
Characterization of the Isolates
Pure colonies of the isolates were characterized based on Cell morphology, gram reaction, KOH test, catalase test, Oxidase test, presence or absence of endospores, motility, oxidation-fermentation test, Fermentation of sugars, H2S production, gas production, Lysine decarboxylase and/or deaminase test, Urease test, Citrate utilization test, Indole test and Methyl red-Voges Proskauer test based on the standard procedure [14].
Antimicrobial Susceptibility Tests for Selected Isolates
Antimicrobial susceptibility test for the bacterial isolates was performed using Kirby Bauer disc diffusion technique for the antibiotics Gentamicin, Erythromycin,Ampicillin, Ciprofloxacin, Oxacillin, and Carbenicillin. Cultures of pure bacterial isolates were inoculated into the nutrient broth and incubated at 37℃ for 24 hrs. The bacterial suspension was adjusted to 0.5 Mc Farland turbidity standards and transferred to Muller-Hinton agar plate using a sterile cotton swab and seeded uniformly by rubbing the swab against the entire agar surface. When the inoculums dried antibiotic-impregnated disks were applied to the surface of the inoculated plates singly. Then, the plates were incubated aerobically at 37℃ for 24 hrs without being inverted. Finally, the zone of inhibition was measured including the disk diameter. The bacterial isolates were categorized as susceptible, intermediate and resistant [15].
Data Analysis
In this study, data were analyzed using Excel and SPSS ver-20 (Turkey’s b) for the bacterial load to tests the significance difference between contamination level among food items and cafeterias at p<0.05.
The Frequency of Bacterial Load on Food Samples
All of the food items contained AMB count in the range of 106 -<107 CFU/g. Particularly, all the samples from “Keyiwot’ and 14 samples from “Tibs” holds the bacterial population in the stated range. However, four samples from “Beyeanet” hold the bacterial population in the range of 104-105 CFU/g (Table 1). According to CFA [15], AMB count from various foods items with<104 CFU/g is satisfactory, 104-105 CFU/g acceptable and>105 CFU/g unsatisfactory. According to this definition, the food items tested in this study were unsatisfactory (Table 1) which is similar to a study conducted in Mekelle city. This high rate of the microbial load could be due to post cooking contamination, inadequate heating, storage at ambient temperature and personal hygiene [16]. In this study, the frequency of AMB count from “Keyiwot”>“Tibs”>”Macaroni”>“Pasta”>“Beyeanet” (Table 1).
Table 1. Frequency distribution of (CFU/g) AMB, AEB and Enterobacteriaceae in food items in cafeterias around WU.
| Food items | No of samples | AMB count (cfu/g) | AEB count (cfu/g) | Enterobacteriaceae Count (cfu/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <105 | 105-<106 | 106-<107 | 103 -<104 | 104-<105 | 105 -<106 | <104 | 104-<105 | 105-<106 | ||
| Beyeanet | 15 | - | 4 | 11 | 1 | 6 | 8 | - | 12 | 3 |
| Keyiwot | 15 | - | - | 15 | 2 | 6 | 7 | - | 11 | 4 |
| Pasta | 15 | - | 3 | 12 | 1 | 11 | 3 | - | 11 | 4 |
| Tibs | 15 | - | 1 | 14 | 4 | 8 | 3 | - | 9 | 6 |
| Macaroni | 15 | - | 2 | 13 | 7 | 8 | - | - | 8 | 7 |
| Total | 75 | - | 10 | 65 | 15 | 39 | 21 | - | 51 | 24 |
Keys: AMB: Aerobic Mesophilic Bacteria; AEB: Aerobic Endospore Bacteria
Similarly, more than 50% of the food samples from “Beyeanet” contain 105-106 CFU/g of AEB. Eleven samples of “Pasta” hold 104-105 CFU/g of AEB. Totally, more than 50% of the food samples hold AEB in the range of 104-105 CFU/g. The dominance of Bacillus spp. from several foods sample attributed to the presence of resistant endospores that give tolerance to adverse conditions and various stresses [17]. 68% of the food samples contained Enterobacteriaceae in the range of 104 -<105 CFU/g (Table 1) in which ≥73% of the food samples were from “Pasta” “Beyeayinet” and “Keyiwot” that was categorized under unacceptable level of the bacterial load as recommended by the New South Wales Food Authority [18]. Such a high count of Enterobacteriaceae was also observed in some street vended foods from Addis Ababa.
The abundance of TCF, FCF and S. aureus in the five food items was in the range of 103-104 CFU/g with percentage food samples of 89%, 79%, and 76%, respectively. The highest average of TCF count was from food sample of “Beyeanet” and “Keyiwot” followed by “Tibs” and “Macaroni”. “Keyiwot” also showed the highest average FCF followed by “Tibs”. Similarly, the highest average S. aureus was from “Beyeanet” and “Tibs” (Table 2).
Table 2. Frequency distribution of (CFU/g) TCF, FCF, and S. aureus in food items in cafeterias around WU.
| Food items | No of samples | TCF count (cfu/g) | FCF count (cfu/g) | S. aureus (cfu/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <103 | 103-<104 | 104-<105 | <103 | 103-<104 | 104-<105 | <103 | 103-<104 | 104-<105 | ||
| Beyeanet | 15 | - | 12 | 3 | 5 | 10 | - | - | 14 | 1 |
| Keyiwot | 15 | - | 12 | 3 | - | 13 | 2 | 5 | 10 | - |
| Pasta | 15 | - | 15 | - | 3 | 12 | - | 7 | 8 | - |
| Tibs | 15 | - | 14 | 1 | 2 | 12 | 1 | 4 | 11 | - |
| Macaroni | 15 | - | 14 | 1 | 3 | 12 | - | 1 | 14 | - |
| Total | 75 | - | 67 | 8 | 13 | 59 | 3 | 17 | 57 | 1 |
Keys: TCF: Total Coliform; FCF: Fecal Coliform
A load of coliforms in the food samples was higher than the standard limit set by NSWFA [19] which categorize them under unacceptable level. The reason for such a high level of contamination could be the long storage time of the prepared food until consumption. Detection of E. coli indicates the recent fecal contamination of the food items; and presence of S. aureus was largely as result of human contact could be poor hygiene practices of the food handlers, as the organism is a normal flora of the human body [20].
The Abundance of Bacterial Groups
The abundance of AMB on the five food items was significantly higher than Enterobacteriaceae on the same food item. However, the abundance of AMB and Enterobacteriaceae was not significantly different from each other (p<0.05) on the same food items. The highest AMB on “Beyeayinet” was 4.923 CFU/g and the lowest was 3.994 CFU/g on “Macaroni” which was significantly different from each other at α=0.05. The highest and lowest TCF count was 3.893 and 3.448 from “Macaroni” and “Pasta”, respectively that was significantly different from each other (Table 3).
Table 3. Mean of different bacterial counts (log CFU/g) from food items in cafeterias around WU.
| Food items | AMB count | AEB count | Enterobacteriaceae count | TCF count | FCF count | S. aureus count |
|---|---|---|---|---|---|---|
| Beyeanet | 6.155a | 4.923b | 4.872b | 3.719cd | 3.166de | 3.692cd |
| Keyiwot | 6.441a | 4.773b | 4.886b | 3.720cd | 3.815c | 3.199de |
| Pasta | 6.293a | 4.453bc | 4.846b | 3.448d | 3.438d | 3.021e |
| Tibs | 6.185a | 4.323bc | 4.998b | 3.853c | 3.445d | 3.329d |
| Macaroni | 6.041a | 3.994c | 4.980b | 3.893c | 3.317d | 3.490d |
Keys: AMB: Aerobic Mesophilic Bacteria; AEB: Aerobic Endospore Bacteria; TCF: Total coliform; FCF: Fecal Coliform. Values designated by the same latter both across column and rows were not significantly different from each other at α=0.05
The highest FCF bacteria (3.815cfu/g) was counted on “Tibs” and the highest S. aureus (3.590cfu/g) was from “Macaroni”. From the food samples, the lowest FCF counted was 3.166 CFU/g from “Beyeanet” (Table 3).
Comparison of Cafeterias for Contamination
The abundance (CFU/g) of AMB, Enterobacteriaceae, and TCF was not significantly different (P<0.05) from each other among the cafeterias although mean of AMB>Enterobacteriaceae>TCF. The highest number of AMB (log) was 4.863 from cafeteria “B” that was significantly higher than AMB from Cafeteria “E” (3.873). The abundance of FCF did not show significant variation among cafeterias. The maximum number of S. aureus was 3.642 from cafeteria “B” that was significantly higher than from Cafeteria “C” at α=0.05 (Table 4).
Table 4. Mean comparison of bacteria (log CFU/g) across sampling cafeterias around WU.
| Sampling Cafeterias | AMB count | AEB count | Enterobacteriaceae | TCF count | FCF count | S. aureus count |
|---|---|---|---|---|---|---|
| Cafeteria ''A'' | 6.253a | 4.444bc | 4.862b | 3.644c | 3.354cd | 3.559c |
| Cafeteria "B" | 6.333a | 4.963b | 4.880b | 3.760c | 3.606c | 3.642c |
| Cafeteria "C" | 6.109a | 4.537b | 4.932b | 3.605c | 3.113cd | 2.940d |
| Cafeteria "D" | 6.126a | 4.650b | 4.962b | 3.791c | 3.520c | 3.447cd |
| Cafeteria "E" | 6.294a | 3.873c | 4.946b | 3.833c | 3.589c | 3.145cd |
Keys: AMB: Aerobic Mesophilic Bacteria; AEB: Aerobic Endospore Bacteria; TCF: Total coliform; FCF: Fecal Coliform. Values designated by the same latter both across column and rows were not significantly different from each other at α=0.05
In this study, a total of 107 bacterial isolates were identified from the foods items and characterized for various genera and bacterial groups. Bacterial microflora were identified as different genera (Table 5). Gram-positive bacteria constituted more than half (52.3%) of the microflora; the majority being Bacillus spp. (25.2%) followed by S. aureus (16.8%). Among the gram-negative bacteria, Alcaligenes (14.2%), Acinetobacter (10.3%), Pseudomonas (7.5%), Aeromonas (6.5%), members of the family Enterobacteriaceae (6.5%) were identified (Table 5) but no Salmonella and Shigella spp. The highest incidence of the microflora 50 (46.7%) was seen in ‘Beyeanet’ with the dominant genera of Alcaligens, Bacillus, Pseudomonas, and Aeromonas. Out of the 50 isolates from ‘Beyeanet ’, 21 (42%) were from cafeteria “B” followed by 17 (34%) from cafeteria “A”. ‘Keyiwot’ harbored 20 (18.7%) of the isolates that was dominated by Bacillus and S. aureus. Out of the 20 isolates from ‘Keyiwot’, 6 (30%) isolates were from cafeteria “D” while five (25%) were each of cafeteria “C” and cafeteria “E”. ‘Pasta’ was ranked third in its microflora load which was mainly dominated by Bacillus and Acinetobacter. Isolates from ‘pasta’ were mainly obtained from cafeterias “E” and “A”.
Table 5. Frequencies of bacterial genera in RTE foods collected from cafeterias around WU.
| Food type by the cafeteria | No of isolates | Gram-positive bacterial results | Gram-negative bacterial results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus spp. | Micrococcusspp. | S. aureus | Other gram +ve rods | Pseudomonasspp. | Aeromonasspp. | Plesiomonasspp. | E. coli | Citrobacter | Alcaligens | Acinetobacter | |||
| Beyenet | Cafeteria | ||||||||||||
| A | 17 | 2 | - | 1 | 2 | 4 | 5 | - | - | - | 3 | - | |
| B | 21 | 3 | 1 | 2 | 1 | 4 | 2 | 2 | 1 | - | 5 | - | |
| C | 6 | 3 | - | - | 2 | - | - | - | - | - | 1 | - | |
| D | 3 | - | - | 1 | - | - | - | - | - | - | 1 | 1 | |
| E | 3 | 1 | - | 1 | 1 | - | - | - | - | - | - | - | |
| Total | 50 | 9 | 1 | 5 | 6 | 8 | 7 | 2 | 1 | 10 | 1 | ||
| Keyi wot | A | 3 | 2 | - | 1 | - | - | - | - | - | - | - | - |
| B | 1 | - | - | 1 | - | - | - | - | - | - | - | - | |
| C | 5 | 1 | - | - | 2 | - | - | - | - | - | - | 2 | |
| D | 6 | - | - | 1 | - | - | - | 1 | 3 | - | 1 | - | |
| E | 5 | 1 | 1 | 1 | - | - | - | - | - | 1 | - | 1 | |
| Total | 20 | 4 | 1 | 4 | 2 | - | - | 1 | 3 | 1 | 1 | 3 | |
| Pasta | A | 5 | 3 | - | - | - | - | - | - | - | - | 1 | 1 |
| B | 1 | - | - | 1 | - | - | - | - | - | - | - | - | |
| C | 3 | 1 | 1 | - | - | - | - | - | - | - | - | 1 | |
| D | 1 | - | - | 1 | - | - | - | - | - | - | - | - | |
| E | 6 | 2 | - | 1 | - | - | - | - | 1 | - | - | 2 | |
| Total | 16 | 6 | 1 | 3 | - | - | - | - | 1 | - | 1 | 4 | |
| Tibs | A | 1 | - | - | 1 | - | - | - | - | - | - | - | - |
| B | 5 | 2 | - | 1 | - | - | - | - | - | - | 2 | - | |
| C | 2 | - | - | - | - | - | - | - | - | 1 | - | 1 | |
| D | - | - | - | - | - | - | - | - | - | - | - | - | |
| E | 2 | 1 | - | 1 | - | - | - | - | - | - | - | - | |
| Total | 10 | 3 | - | 3 | - | - | - | - | - | 1 | 2 | 1 | |
| Macaroni | A | - | - | - | - | - | - | - | - | - | - | - | - |
| B | 7 | 4 | - | 1 | - | - | - | - | - | - | - | 2 | |
| C | - | - | - | - | - | - | - | - | - | - | - | - | |
| D | 1 | - | - | 1 | - | - | - | - | - | - | - | - | |
| E | 3 | 1 | - | 1 | - | - | - | - | - | - | 1 | - | |
| Total | 11 | 5 | - | 3 | - | - | - | - | - | - | 1 | 2 | |
| Overall | 107 | 27 | 3 | 18 | 8 | 8 | 7 | 3 | 5 | 2 | 15 | 11 | |
‘Tibs’ was the least in bacterial load and 50% of them were from cafeteria “B”. Similar bacterial isolates were also identified from RTE foods in common retail shops, restaurants, and abattoir of Jimma City in almost the same types and proportion [16,21]. However, Salmonella and Shigella spp. were not identified in this study that could be the hand washing habit of the workers after the toilet.
Antimicrobial Susceptibility Tests for Representative Bacterial Genera
Ten bacterial isolates were tested for anti-microbial susceptibility test, of which 7 (70%), 5 (50%), 3(30%), 10(100%), 0 (0%) and 4 (40%) were susceptibility to Gentamicin, Erythromycin,Ampicillin, Ciprofloxacin, Oxacillin, and Carbenicillin, respectively. In other words, Bacillus spp, Aeromonas, and Alcaligenes were intermediates to Gentamicin, Erythromycin, andAmpicillin, respectively. S. aureus was resistant only to Oxacillin whereas Plesiomonas spp. was susceptible only to Ciprofloxacin, and the other were found to be in between of the two showing resistance to more than one of the tested antimicrobials (Table 6).
Table 6. Antimicrobial susceptibility tests for some bacterial isolates.
| Gentamicin (10 g/ml) | Erythromycin (15 g/ml) | Amicillin (10 g/ml) | Ciprofloxacin (5 g/ml) | Oxacilling (1 g/ml) | Carbenicillin (100 g/ml) | |
|---|---|---|---|---|---|---|
| Bacillus spp | I | S | R | S | R | R |
| S.aureus | S | S | S | S | R | S |
| Micrococcusspp | S | S | S | S | R | S |
| Pseudomonasspp | S | S | R | S | R | S |
| Plesiomonas | R | R | R | S | R | R |
| E. coli | S | R | R | S | R | R |
| Alcaligenes | R | R | I | S | R | R |
| Aeromonas | S | I | R | S | R | R |
| Acinetobacter | S | S | S | S | R | S |
| Citrobacter | S | R | R | S | R | S |
S=Susceptible, R=Resistant, I=Intermediate
In this study even though pathogenic microorganisms like Salmonella and Shigella were not identified, the resistance pattern in bacterial isolates from RTE foods indicates that there might be fast-growing public health threat within the community. Similar results were observed on studies done in different parts of Ethiopia [8,21].
This study tested for the abundance of the bacterial group in RTE foods items collected from different cafeterias around WU. Representative isolates from bacterial groups were tested for resistance to different antibiotics. The food items hold a high abundance of AMB, AEB, Enterobacteriaceae, TCF, FCF, and S. aureus but not the pathogenic bacteria like Salmonella and Shigella. The abundance of the bacterial group varies among the bacterial group, food items, and a cafeteria. The representative bacterial group showed variation for resistance to antibiotics and the isolates were sensitive to ciprofloxacin. In general, an abundance of the bacterial isolates on RTE foods sampled round WU were beyond the acceptable level of the microbiological guideline/standards. The high microbial load in RTE food items could be associated with the poor personal and sanitary hygienic practice. Therefore, continuous training for the food handlers and inspection of the sanitary practice in the cafeterias could improve the microbiological quality of RTE foods around the university.