e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Chemistry and Chemical Engineering, Luliang University, Lvliang, PR China
2Graduate Institute of Pharmaceutical Chemistry, Luliang University, Lvliang, PR China
Received date: 24/06/2016; Accepted date: 01/07/2016; Published date: 06/07/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
Deuteration of anilines, I/D exchange, D2O.
Isotopic labelling is a technique, which has been widely used in chemistry and biochemistry to help understand chemical reactions and interactions, as well as stable isotopes in pharmacokinetic studies as biological internal standards. Usually, D2O is the cheapest deuterium source, so far [1] several methods have been developed for the synthesis deuterium-labelling compounds through H/D exchange with D2O, deuterium gas and deuterium solvents. The common pathways are pH-dependent H/D exchange [2-6] and metal catalyzed H/D exchange [7-13]. Most of these methods have some disadvantages, such as time consuming, lower efficacy, very high reaction temperature, and expensive deuterium source, as well as precious metal was used as catalyst in most of these cases. Other methods for achieving deuterium-labelling compound through including decarboxylative deuteration, I/D exchange. It is well-known that deuterium-labelling anilines are a type of important intermediate due to its good reactivity, and a few of methods for synthesizing deuterium-labelling anilines have been reported. In 2008, Mutsumi et al. [14] reported a tributyltin hydride promoted I/D exchange reaction for deuteration on pyrimidine and purine nuclei with THF-d8 as deuterium source. After that, Lautens et al. [15] developed a palladium mediated coupling-reductive method for obtaining meta-substituted biaryls. In both above mentioned method for deuterating aromatic compounds are highly expected. On the other hand, high-speed microwave synthesis has attracted a considerable amount of attention in the last two decades. Compared with conventional heating, microwave irradiation displayed a number of advantages, not only in heating effect, but also good selectivity and higher yield in many microwave promoted reactions [16,17]. Herein, we would report a microwave promoted I/D exchange method for getting deuterated aniline with D2O as an inexpensive deuterium source.
General information
Unless otherwise noted, commercial reagents were used as received. 1H (400 MHz) and 13C (100 MHz) NMR chemical shifts were reported in CDCl3 7.27 ppm for 1H, 77 ppm for 13C as standards and coupling constants(J) are reported in hertz (Hz). The following abbreviations are used to designate signal multiplicity: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, br=broad.
General procedure
In a 25 ml of seal tube, aniline (2 mmol), D2O (3 ml) and SOCl2 (0.2 ml) was added successively. Then this tube was irradiated under microwave at 130°C for 30 min. After cooled to room temp., the reaction was diluted with water, and neutralized with NaHCO3, extracted with diethyl ether (50 mL × 3), the combined organic layer was washed with brine, and dried with anhydrous Na2SO4. Removal of all volatiles by vacuum evaporation left a residue, which was purified by flash chromatography to afford product.
2-deuterium-4,6-dimethylaniline (2a). Colorless oil; 1H NMR (400 MHz, CDCl3): δ 6.86 (s, 1H), 6.83 (s, 1H), 3.44 (br s, 2H), 2.22 (s, 3H) and 2.13 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 141.98, 131.15, 127.87, 127.27, 122.50, 114.93 (1:1:1 t, J=23.5 Hz), 20.45, 17.33; HRMS (EI) calcd. for C8H10DN [M+] 122.0954, found 122.0955; IR (KBr): cm-1: 3266, 2920, 2866, 1687, 1635, 1510, 1482, 1300, 880.
4-deuterium-2,6-dimethylaniline (2b). Colorless oil; 1H NMR (400 MHz, CDCl3): δ 6.94 (s, 2H), 3.57 (br s, 2H) and 2.81 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 142.66, 128.23, 121,86, 117.92 (1:1:1 t, J=24.5 Hz), 17.69; HRMS (EI) calcd. for C8H10DN [M+] 122.0954, found 122.0956; IR (KBr): cm-1: 3260, 2960, 2850, 1730, 1620, 1545, 1260, 1080, 1025, 970, 803, 662.
2-deuterium-4-phenyl-6-methylaniline (2c). Colorless oil; 1H NMR (400 MHz, CDCl3): δ 7.54-7.52 (m, 2H), 7.40-7.36 (m, 2H), 7.31-7.22 (m, 3H), 3.63 (br s, 2H) and 2.22 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 144.07, 141.39, 131.70, 129.26, 128.66, 126.50, 126.20, 125.59, 122.59, 115.00 (1:1:1 t, J=23.5 Hz), 17.55; HRMS (EI) calcd. for C13H12DN [M+] 184.1111, found 184.1115; IR (KBr): cm-1: 3330, 3322, 3000, 2941, 2920, 2830, 1620, 1480, 1300, 1267, 1081, 1030, 897, 770, 699.
2-deuterium-4-methyl-6-phenylaniline (2d). Yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.45-7.40 (m, 4H), 7.34-7.30 (m, 1H), 6.96-6.95 (m, 2H), 3.55 (br s, 2H) and 2.27 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 140.94, 139.73, 131.00, 129.12, 128.95, 128.78, 127.88, 127.78, 127.11, 115.56 (1:1:1 t, J=24 Hz), 20.45; HRMS (EI) calcd. for C13H12DN [M+] 184.1111, found 184.1117; IR (KBr): cm-1: 3452, 3350, 2922, 1623, 14389, 1265, 872, 779, 742, 698, 587.
2-deuterium-6-bromo-4-methylaniline (2e) Yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.23 (s, 1H), 6.90 (s, 1H), 3.92 (br s, 2H) and 2.22 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 141.52, 132.74, 129.09, 128.92, 115.54 (1:1:1 t, J=24.5Hz), 109.34, 20.09; HRMS (EI) calcd. for C7H7BrDN [M+] 185.9903, found 185.9904; IR (KBr): cm-1: 2926, 2848, 1730, 1507, 1465, 1269, 1080.
2,6-dideuterium-4-methylaniline (2f) Yellow oil; 1H NMR (400 MHz, CDCl3): δ 6.96 (s, 2H), 3.51 (br s, 2H) and 2.24 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 143.72, 129.66, 127.79, 115.01 (1:1:1 t, J=23.5 Hz), 20.47; HRMS (EI) calcd. for C7H7D2N [M+] 109.0861, found 109.0866; IR (KBr): cm-1: 3290, 2970, 2930, 2870, 1734, 1678, 1600, 1477, 1274, 1203, 1090, 985, 905, 775, 502.
2,4,6-trideuteriumaniline (2g) [6] Yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.15 (s, 2H) and 3.62 (brs, 2H).
1-(4-amino-3,5-dideuterophenyl)-2,2,2-trideuteroethanone (2h) [17] Yellow oil; 1H NMR (400 MHz, CDCl3): δ 7.79 (s, 2H) and 4.33 (br s, 2H).
Our work was initialed by using 2-iodo-4, 6-dimethylaniline 1a as substrate, which was refluxed in CD3OD in the presence of thionyl chloride for 12 h, 2-deuterium-4,6-dimethyl -aniline was obtained at 20% yield (Table 1). When CD3OD was replaced with cheaper D2O, and the reaction was refluxed for 12 h, I/D exchanged product was obtained in 24% yield (Table 1). Further prolonging the reaction time could not increase the yield (Table 1). Next, the microwave irradiation was applied to promote this reaction, to our great delight, when 4-iodo-2,6-dimethylaniline was heated in a sealed tube by microwave irradiation with D2O in combination with SOCl2 at 100°C for 15 min., I/D exchanged product was obtained in 56% yield (Table 1). When the reaction time was prolonged to 30 min., 84% yield was obtained (Table 1).
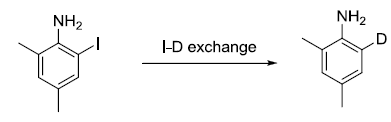
| Entry | Solvent | Reaction condition | Yield(%)b |
|---|---|---|---|
| 1 | CD3OD/SOCl2 | reflux, 12 h | 20 |
| 2 | D2O/SOCl2 | reflux, 12 h | 25 |
| 3 | D2O/SOCl2 | reflux, 24 h | 26 |
| 4 | D2O/SOCl2 | MW(100°C, 15 min) | 56 |
| 5 | D2O/SOCl2 | MW(100°C, 30 min) | 84 |
Table 1: Optimization of the reaction conditionsa
With this optimized reaction condition, other iodine substituted anilines were also extended. Most of these iodine substituted anilines could be transferred to corresponding I/D exchanged products with moderate to good yield (Table 2). The Br/D exchange be observed, singe I/D exchanged product and double deuterium product were obtained (Table 2) led to lower yield of 2e. As shown in Table 2, I–D exchange reaction of all reactants to achieve a high D content.
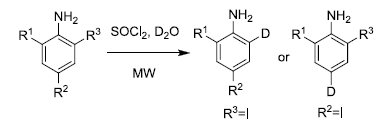
| Entry | Substrate | R1 | R2 | R3 | Product | D contenta (%) | Yield (%)b |
|---|---|---|---|---|---|---|---|
| 1 | 1a | Me | Me | I | 2a | 99 | 84 |
| 2 | 1b | Me | I | Me | 2b | 98 | 80 |
| 3 | 1c | Me | Ph | I | 2c | 98 | 85 |
| 4 | 1d | Ph | Me | I | 2d | 98 | 85 |
| 5 | 1e | Br | Me | I | 2e | 97 | 26 |
| 6 | 1f | I | Me | I | 2f | 99 | 73 |
| 9 | 1g | H | H | I | 2g | 98 | 67 |
| 10 | 1H | H | Ac | I | 2h | 98 | 80 |
aDetermined by 1H NMR spectroscopy in CDCl3
bIsolated yield
Table 2: I/D exchange on aniline using D2O as a deuterium source.
The possible mechanism can be proposed as Scheme 1. First, thionyl chloride reacted with D2O to produce DCl in situ, which act as an electrophile. The amino group in aniline is strongly activating and ortho/para-directing group, when the electrophile DCl attacks the ortho positions of aniline, the nitrogen atom can donate electron density to the π system to form an iminium ion, then chloro ion attacksiodine to form ICl.
In conclusion, a microwave promoted I/D exchange deuteration of anilines method was developed. Under microwave condition, several iodo anilines could be rapidly and efficiently deuterated in a short time with D2O as a deuterium source.
We are grateful to Shanxi Natural Science Foundation Committee (Project No.: 2012011002-3), Shanxi High School Development Project of Science and Technology Research (Project No.: 20101154; 2013168), Luliang University Science and Research Foundation (Project No.: ZRXN201211). The authors are also very grateful for the English and manuscript suggestions by Prof. Bai, J.M. and Bogan, A. from department of foreign language, Luliang University.