e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
1Department of Physical Chemistry of Polymers, PetruPoni Institute of Macromolecular Chemistry, Iasi, Romania
2Department of Physical Chemistry and Materials Science, Budapest University of Technology and Economics, Budapest, Hungary
3Sciences Department, Faculty of Physics, Al. I. Cuza University, Iasi, Romania
4Product Design, Mechatronics and Environment Department, Transilvania, University of Brasov, Brasov, Romania
5Food Safety Department, Veterinary Laboratory and the Food Safety, Iasi, Romania
Received date: 30/05/2015 Accepted date: 10/07/2015 Published date: 13/07/2015
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
Elaboration of a two steps physico-chemical process to obtain antimicrobial and antioxidant materials. A two-step process was used for modification of a cellulose/chitin mix fiber (CC)first an enzymatic activation with a commercial cellulase enzyme was applied and then a coupling reaction with phenolic antioxidants such as p-hydroxybenzoic acid (HBA),gallicacid(GA) and eugenol (Eu) was carried out to evolve antimicrobial and antioxidant properties. Both the enzyme activated and the subsequently modified with antioxidant moieties samples were characterized by ATR-FTIR spectroscopy, X-ray photoelectron spectroscopy scanning electron microscopy and thermogravimetry. Furthermore, the antioxidant and antibacterial capacities were tested. All obtained results confirm the morphological and structural changes on the fiber surface after the twostep procedure of modification. Based on the XPS results, the modification degrees of the superficial layers of the fibers varied between 35 and 43%. Both the antimicrobial and radical scavenging activities were up to 100% and the order of the antioxidant activity can be written as: CC/Eu > CC/ GA > CC/HBA. Materials with these functionalities can be widely applied in the dermo-cosmetic field to protect the skin from oxidative stress, as transdermal patches which could be effectively used as a material in wound dressings.
Antioxidant, Antimicrobial, ATR-FTIR spectroscopy, Nucleic acids, Chemical treatment.
From the many kinds of polysaccharides, cellulose and chitin are the most important biomass resources [1]. While cellulose fibers are moisture-absorbent and comfortable, chitin fibers show biostatic, inflammation diminishing, odor-resistant, odorpreventing and itch-resistant properties [2]. Thus, the combination of cellulose with chitin is an attractive task for producing materials with combined and/or special properties [3,4]. Cellulose is used in a wide range of applications including composites, netting, upholstery, coatings, packing, paper, etc. Chemical modification of cellulose is performed to improve processability and to produce cellulosic materials which can be tailored for specific industrial applications. In general, cellulosics are strong, reproducible, recyclable and biocompatible, being used in various biomedical applications [5].
Textiles containing antioxidants may permit a similar degree of skin diffusion as the transdermal patches used in the pharmaceutical field [6]. Cellulose is insoluble in water and also in most of the common solvents. The poor solubility is attributed primarily to the strong intramolecular and intermolecular hydrogen bonding between the individual chains. For its modification, various procedures have been applied. Different approaches have been used for antimicrobial functionalization of textile materials avoiding those occurring in organic solvents as physical procedures as plasma activation [7,8].
Antimicrobial agents can be incorporated into the fibers [9], applied on the fiber surface [10], or chemically bonded onto the fibers [7,11]. Antioxidants are natural substances used to regulate the processes triggered by external aggressions, thereby preventing the oxidative stress. Natural bioactive agents with antimicrobial properties have become increasingly important for biofunctionalization of textile fibers, because they enable the production of safe, non-toxic, skin- and environment-friendly bioactive textile products. Plant-based products represent the major group of antimicrobial agents, which consist of substances such as phenols (simple phenols, phenolic acids, quinines, flavonoids, flavones, tannins and cumarines), terpenoids, essential oils, alkaloids, lectines, polypeptides and polyacetylenes. These components show, not only antimicrobial, but also antioxidant properties. This is extremely important when developing innovative biomaterials for medical applications, such as bioactive dressings and wound-healing isolation materials. For these kind of applications, besides antimicrobial inhibition, it is very important to provide a reduction in those reactive oxygen species that are strongly implicated in the pathogenesis of wounds, causing injury with bio-molecules such as lipids, proteins and nucleic acids [12].
Antimicrobial agents can act in two distinct ways: (a) by contact; the antimicrobial agent is bound onto the fiber surface, and (b) by diffusion; the antimicrobial agent is slowly released onto the fiber surface and/or from the surface. The main advantage of bound antimicrobial agents is that they do not leach-off the textile substrates into the surroundings, so the probability of microorganisms developing resistance to them is small. Since the bound antimicrobials are firmly attached onto the fiber surface, they are more durable to laundering than leaching antimicrobials. In the case of controlled-release mechanism, the substances are not chemically-bonded on the textile surface and the 'reservoir' of agent to be delivered is limited, thus, the concentration of active agent decreases eventually and gradually falls under the limit of effectiveness, which can consequently induce resistance to these substances in microorganisms [13].
Phenolic compounds are well known as antioxidants against superoxide radical. Phenols are known to reduce the rates of oxidation of organic maters by transferring a H atom (from their OH group) to the chain-carrying ROO radicals [14]. Several phenolic compounds exerting antioxidant and antimicrobial activity have been used to modify polymeric materials. They can react with enzymes to different extents and therefore potentially be grafted on the cellulosic fiber surface to develop covalently bound antioxidant fiber based products [15].
It is extremely hard to remove the biofilms formed on the surface, since the adhered bacteria show a great resistance to all kinds of biocides. Thus, it is crucial to design surfaces which will not allow the settlement of microbes at the very first place. Thus, keeping this strategy in mind, antimicrobial surfaces are designed with the principle that they should either repel the microbes or kill them on contact without causing skin sensitization [16].
In this study, p-hydroxybenzoic acid (HBA), gallic acid (GA) and eugenol (Eu) were used as reference antioxidant and antimicrobial agents for the modification of cellulose/chitin mix fibers (CC) using enzyme activation followed by a coupling reaction. The optima conditions for the fiber surface modification were established in order to obtain materials for pharmaceutical and medical applications such as transdermal patches for wound dressing or cosmeto-textiles.
Materials
The unwoven cellulose/chitin mix fibers delivered under the trade name of CHITCEL by Shandong, (China) contain 9 – 11 wt% chitin, which imparts antimicrobial properties to fibers. Celluclast 1.5 L enzyme, a cellulase mixture produced by Trichoderma reesei (83 FPU/ml) from Sigma-Aldrich. was used for activation of the fibers. The enzyme was used as received. Gallic acid, p-hydroxybenzoic acid and eugenol were purchased from Sigma-Aldrich. The purity was of 99% for p-hydroxybenzoic acid and eugenol and 98% for gallic acid, and they were used without further purification.
Procedure of Cellulose/Chitin Mix Fibers Modification
The cellulase enzyme treatment of the cellulose/chitin mix fibers was carried out with 1 g/l Celluclast 1.5 L at 50°C for 1 hour at pH5 (0.05 M acetate buffer). The fiber-to-enzyme solution ratio was 1:10 (w/w). After the incubation period, the enzyme was deactivated by washing the fiber samples in distilled water at 90°C for 5 minutes. Then they were rinsed thoroughly twice in hot water and twice in cold water, centrifuged for 20 minutes at 8000 rpm in order to remove the excess water, and finally air dried.
After enzyme activation the fibers were immersed in solutions of p-hydroxybenzoic acid, gallic acid and eugenol,7.5 g/L concentration (the solutions were made using the same buffer solution as for the enzyme activation), for 4 hours, at room temperature, at a vigurous stirring (~120 rpm). Then the fibers were centrifuged for 15 minutes at 8000 rpm in order to remove the excess of reactant. After modification, the fibers were extracted in a Soxhlet extractor with chloroform for samples modified with p-hydroxybenzoic acid and eugenol, and with methanol for gallic acid, for 6 hours, in order to remove the physical absorbed and unreacted chemicals. The modified fibers (CC/HBA,CC/GA and CC/Eu) were then air dried and analyzed.
Attenuated Total Reflection - Fourier Transform Infrared Spectroscopy (ATR-FTIR)
ATR-FTIR spectra were recorded at 4 cm-1 resolution with 64 scans by means of a spectrometer Bruker VERTEX 70, in absorbance mode, by the ATR technique with a 45° ZnSe crystal. Penetration thickness was about 100 microns. For each sample, the evaluations were made on the average spectrum obtained from three recordings. Background and sample spectra were obtained in the 500 to 4000 cm-1 wavenumber range. The processing of spectra was achieved using a SPECVIEW program.
The X-Ray Photoelectron Spectroscopy (XPS)
XPS measurements were made using a PHI 5000 Versa Probe spectrometer (ULVAC-PHI,INC) equipped with a monochromatic Al Kα X-ray source (λ = 14,866 eV). The pressure in the analysis chamber was kept at 2 x 10-6 Pa or lower during each measurement. Measurements were taken at a take-off angle of 45° with respect to the sample surface. The MultiPak V8.2C software was used for background subtraction, peak integration, fitting and quantitative chemical analysis.
Scanning Electron Microscopy (SEM)
SEM Analysis was taken with a scanning electron microscope SEM/ESEM – EDAX QUANTA 200, without any further treatments, at a magnification of 10,000X.
Thermogravimetric Analysis (TGA)
TGA was carried out on a DTA/TG coupled instrument, model STA 449 F1 Jupiter (Netzsch,Germany) under constant nitrogen flow (200 mL/min) at a heating rate of 10 ºC/min. Heating and cooling runs were performed at a 10°C/min rate.
DPPH Radical Scavenging Assay
The radical scavenging activity of cellulose/chitin mix fibers was measured using the adapted stable radical 2,2-diphenyl-1- picrylhydrazyl (DPPH) method. Briefly, approximately 30 mg of fiber was placed in a flask /containing 2 mL of methanolic solution of DPPH (0.06 mM) and was vortexed vigorously for 1/2 h at room temperature (20°C) in the dark. The phenolic compounds grafted onto surface of modified fibres will react with DPPH.The remaining DPPH was determined by absorbance at 517 nm using a UV spectrometer (Cary 60 UV-Vis Spectrophotometer). The control was obtained using the same procedure but mixture contained the unmodified fibres. The radical scavenging activity (RSA) of the cellulose/chitin mix fibers was calculated according to equation (1)
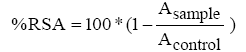 (1)
(1)
Where: Asample represents the absorbance of the sample solution and Acontrol represents the absorbance of DPPH solution with the addition of the unmodified fibers.
Two series of antimicrobial tests were applied. The first one was effectuated according to standard methods SR ISO 16649- 2/2007–Microbiology of alimentary and animal products. The experimental protocol for testing antimicrobial efficiency against Escherichia coli, consists in the following stages:
sterilization of medium for bacteria growth (without or with samples); TBX Chromogenis agar, based on Tryptone Bile Salts Agar medium has been used to detect and enumerate E. Coli; the addition of x-ß-D-Glucuronide, as chromogenic agent, allows detection of the presence of the enzyme glucuronidase, which is highly specific for E. coli. This bacteria absorb the chromogenic agent x-ß-D-glucuronide, and the intracellular glucuronidase enzyme activity breaks the bond between the chromophore and the glucuronide. The released chromophore is colored and builds up within the cells, causing the E. coli colonies to be blue-green colored. [SR ISO 16649 (E. coli; “Horizontal method for β-glucuronidase-positive Escherichia coli - Part 2: Colony count technique at 37°C using 5-bromo-4-chloro-3-indolyl β-D-glucuronide”) according to “Minerals Modified Glutamate Broth” (Catalog 1365)]. Sterilization of the culture medium was made in an autoclave at 110 °C and 0.5 bar for 20 min.
Obtaining of the ATCC culture
was done by seeding 0.1 mL of primary culture on the medium (with and without sample);
Inoculation and incubation performed for 24 and 48 hours at 37 °C;
Identifying and count the target germs
The colonies obtained by selective culture medium separation, have been count, both on neat culture medium and on culture medium with samples, by using a Funke Gerber Colony Star bacteria counter.
5- Calculation of the inhibition efficiency by using the equation (2)
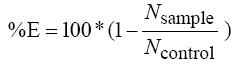 (2)
(2)
Where:
E is the inhibition efficiency (%)
Nsample is the number of colonies developed on the growth medium containing the tested fibers
Ncontrol is the number of colonies developed on the growth medium
In the second series, E. coli test determines qualitatively the colonies of E. coli developed around or over the sample surface. It uses a reference stock culture to obtain pure populations of individual microorganisms with known and predictable growing demands.
Obtaining primary culture
Kwik-STIK E.Coli ATCC 25922 unit was thermally equilibrated at room temperature, removed and sticked to the primary agar plate for identification. The gelatin tablet has been hydrated and the inoculation material is transferred on the agar medium with nonselective growth (primary culture). The inoculated area is stroke off to obtain isolated colonies. The medium is immediately incubated, which determines the selection of representative well insulated colonies, to be used for transfers in Petri plates where the fiber samples will be applied.
Obtaining the secondary culture
sOn the seventh day since processing reference strain for obtaining primary culture, a test culture in nonselective agar medium is prepared: representative, well isolated colonies from primary culture are dispersed on the medium, inoculating a circular area with a diameter of 25 mm and then stroke off 10-20 times the inoculated area to get isolated colonies. The subculture is then incubated for 24 hours at 37°C.
Inoculation
The previously obtained subculture is used for seeding the Petri plates in which SOY AGAR Tryptone medium was previously introduced. A sample of fiber was placed in each Petri box. They were incubated for 24 h at 37°C. After incubation, the developing of bacteria colonies around or attached to the fiber sample was qualitatively (visual) evaluated. If the culture medium remains transparent around the sample, a bacteriostatic effect of the fiber could be assumed. After fiber removal from the medium and its new submission for incubation, without the sample and keeping the same conditions, a new evaluation of the bacteria growth has been made. If bacteria developed on the place where initially the sample has been positioned, it could be assumed that the polymer has only bacteriostatic effect. If there are no bacteria developed on the samples’ place after the new incubation period, a bactericide effect of the sample is considered to be active.
ATR-FTIR Spectra Results
The ATR-FTIR spectra of the fibers contain the main infrared spectral bands of the cellulose and chitin, the components of the mix fibers, and also bands appearing after enzymatic activation and subsequent modification with p-hydroxybenzoic acid, gallic acid and eugenol (Figure 1 and Table 1).
The following spectral characteristics have been evaluated:
The energy of the H-bonds has been calculated using equation (3) [17].
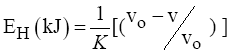 (5)
(5)
Where: νo - standard frequency corresponding to free –OH groups (3650 cm-1)
ν - The frequency of the bonded –OH groups; k = 4*10-3 kJ-1
The enthalpy of H-bond formation has been evaluated using equation (4) [18].
ΔH (kJ / mole) = 0.0672ΔvOH + 2.646 (4)
Where: Δ νOH - OH wave number shift (cm-1)
The asymmetric index (a/b) is ratio between peak full width at half height of the OH absorption band [19].
The obtained data are summarized in Table 2.
Useful comparison between the survey spectra revealed that the C and the O are the predominant species (they are usually found at cellulose/chitin mix fibers surface) and, as expected, new characteristic O and N emission peaks appeared in modified cellulose/chitin mix fibers samples spectra due to enzyme and/or chemical treatment Figure 2.
The most evident change in the fiber structure, or at least on the surface of the fiber, can be detected in the number and type of carbon atoms. Both untreated and enzyme treated fibers have four types of carbon atoms: C1 - a carbon atom bonded only to another carbon (C-C), or to a hydrogen atom (C-H); C2 - a carbon atom bonded to an oxygen atom (C-O from C-OH), to a nitrogen atom (C-N), or from C-O-C group; C3 - a carbon atom single bonded of two oxygen atoms (-O-C-O-) or to a single carbonyl atom (-C=O) or a single bonded to a nitrogen atom and double bond to an oxygen atom (N-C=O); C4 - a carbon atom single bonded to an oxygen atom and to a carbonyl oxygen atom (O-C=O).
The binding energy and percentage area of deconvoluted carbon peaks for cellulose/chitin mix fibers, untreated and modified are listed in Figure 3.
The oxygen deconvoluted peaks evidenced two types of oxygen atoms: O1: an oxygen atom linked to a carbon atom by single bond (C-O-) or a hydroxyl oxygen (OH); O2: an oxygen atom bonded to a single carbonyl atom (-C=O), single bonded of two carbon atoms (C-O-C), or a hydroxyl oxygen atom bonded to a carbon atom (C-OH).
The binding energy and relative concentration of deconvoluted oxygen peaks for untreated and modified cellulose/chitin mix fibers are summarized in Figure 4.
The surface morphology of untreated and modified fibers was analysed by SEM – Figure 5.
DPPH radical scavenging assay
The radical scavenging activity of cellulose/chitin mix fibers before and after modification with phenolic compound was determined and the results are presented in Table 3.
The radical-scavenging activities of phenolic acids depend on the number of hydroxyl moieties attached to the aromatic ring of the benzoic acid molecule. Gallic acid, with three hydroxyl groups, was observed to be more active than p-hydroxybenzoic acid. Also, methoxy moieties attached to the aromatic ring increases the radical-scavenging activity; that is why eugenol was more active than gallic acid and p-hydroxybenzoic acid [20].
Antimicrobial tests
The results of antimicrobial tests for inhibition of Gram-negative Escherichia coli are given in Table 4.
The effect of the antimicrobial content on the material can also be evaluated qualitatively by taking into account the bacteria growth on the nutritive agar plate. In Figure 6 the representative images corresponding to E. coli inoculation are presented.
The inoculation of nutritive agar plates with selected bacterial strains followed by the disposal of modified fibers allows inhibiting cell growth on the fiber area (Figure 6).
Figure 6: Antimicrobial activity of the test specimens assessed against E. coli by the qualitative method: (a) CC fibres and representative image for CC/phenolic antioxidant fibers (b) incubated for 24 h at 37°C and (c) re-incubated after fiber removal from the medium and its new submission for incubation.
Some new bands appear in the ATR-FTIR spectra of enzymes activated fibers (Figure 1a) namely:1561 cm-1 (assigned to C=O stretching vibrations); 1411 cm-1 (assigned to C-OH group, C-OH in plane bending), 1268 cm-1 (assigned to CONHR group, Amide III, C-N stretching vibrations and NH deformation vibrations). By enzyme activation, the inner layers of the fibers which contain more chitin are revealed and provide C – N and NH links.
Some bands appearing in the ATR-FTIR spectra - Figure 1b - correspond to p-hydroxybenzoic acid 1560 cm-1 (assigned to COOH group, asymmetric C=O stretching vibrations) or to the new links that were formed between cellulose/chitin mix fibers and p-hydroxybenzoic acid: 1263 cm-1 (corresponding to Aryl-COO- group, C-O stretching vibrations). Also from Figure 1c some new bands can be observed that correspond to gallic acid: 1560 cm-1 (assigned to COOH group, asymmetric C=O stretching vibrations), or to the new links that were formed between cellulose/chitin mix fibers and gallic acid: 1264 cm-1 (corresponding to - Aryl-COOgroup, C-O stretching vibrations). New bands are also identified that correspond to eugenol: 1560 cm-1 (assigned to asymmetric C=O stretching vibrations), or to the new links that were formed between cellulose/chitin mix fibers and eugenol: 1265 cm-1 (assigned to asymmetric C-O-C stretching vibrations) – (Figure 1d).
There are also important shifts of the bands position to lower or higher wave numbers or split of different bands, which indicate that the new compounds are formed after two step treatment process – (Table 1). Based on these results it can be concluded that the modification took place after activation by enzyme treatment.
After modification, one can observe (Table 2) an increase of the asymmetric index from 0.75 to 0.88 for CC/HBA, to 0.85 for CC/GA and to 0.87 for CC/Eu, and an increase of the hydrogen bonding energy from 20.27 kJ to 21.44 kJ for CC/HBA, to 21.58 kJ for CC/GA and to 21.44 kJ for CC/Eu, and a decrease of the hydrogen bond enthalpy, which should means that the structural order has been modified.
The XPS data showed that the percentage of oxygen detected was higher on the surface of all cellulose/chitin– modified fibers (37.8% for CC/HBA, 34.3% for CC/GA and 32.2 for CC/Eu) than on that of the untreated ones (27.9%), while the percentage of carbon atoms is lower on the surface of all cellulose/chitin–modified fibers (61.8% for CC/HBA, 64.9% for CC/GA and 66.7% for CC/Eu) than on that of the untreated ones (72.1%).
After modification, the O/C ratio increased for all enzymes - activated fibers, suggesting that the surface modification occurred. No amount of nitrogen was found on the surface of untreated fibers. Nitrogen can be detected only on the surface of the enzyme activated fibers and the subsequently treated samples (3.4% for CC/HBA,4.5% for CC/GA and and 5.8% CC/Eu) and it can arise from chitin more available at surface after enzyme activation.
No significant changes in binding energies were observed for deconvoluted carbon peaks of the modified samples. For all samples, the percentage of the C1 peak decreased after modification; also, the C2 peak suffers modification. A possible explanation is that through enzymatic treatment new links are formed and simultaneously the surface of the fiber is activated. Thus, the chitin can also interact providing C - N links. Furthermore, the C3 and C4 peaks showed an increase for all modified samples. The C4 peak was in a small percentage in all samples most likely due to the low concentration of carboxylic groups on the surface of the fibers.
The hydroxyl group content in the superficial layers of the cellulose/chitin mix fibers is 32.69%.The C2 groups (C–O– from C–OH) suffer variations for all modified samples. C3 groups also increases significantly from 17.44% to 48.16%. Based on the above values, a modification degree of the surface layers can be estimated at about 42.45% for CC/HBA, 36.92% for CC/GA, 35.43% for CC/Eu. The greater reactivity of the HBA and GA could be explained by the presence of carboxylic groups, which can also participate in the reaction with active sites onto activated cellulosic surface. Gallic acid and p-hydroxybenzoic acid have been successfully grafted onto the fibre surface significantly, increasing the number of carboxylic acid groups in accordance with results obtained by Chandra et al. [21].
In addition, the oxygenated to unoxygenated carbon ratio (Cox/Cunox) was also calculated with the following
Equation 5
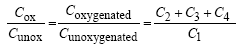 (5)
(5)
The XPS data listed in Figure 3 showed an increase in the total oxygenated carbon bond, the Cox/Cunoxalso suggesting that surface modification occurred.
O1 peak showed a decrease after modification by enzyme activation. At the same time, following modification, the O2 peak which is characterized by a higher binding energy showed an increase caused by the new links created during enzyme treatment.
Nitrogen was found only on the surface of the activated and modified samples, since partial degradation of cellulose is more pronounced at surface. The nitrogen deconvoluted bands are being evidenced by two types of nitrogen atoms for enzyme treated samples with binding energy and relative concentration given in Table 4. N1: a nitrogen atom linked by a single bond to two carbon atoms C-NH-C or a nitrogen atom linked to a single carbonyl atom (N-C=O); N2: a nitrogen atom linked to a carbon atom by a single bond (C-N). The value of the N1 peak is higher than that of the N2 peak for all modified samples.
From SEM images, one can observe that the surface of untreated fibers was quite homogenous and that the individual fibers were intact (Figure 5a). Figures 5b-d illustrates the action of modification treatment with HBA, GA and Eu, evidences on the fiber surface, fibers showing a rougher surface, and also a thin layer of deposits, which apparently covers the whole surface.
Modified cellulose/chitin mix fibers showed radical scavenging activity up to 5.54% for CC/HBA, 16.26% for CC/GA and 100% for CC/Eu (Table 3). The antioxidant activity is function of the phenolic compound used. Results proved that eugenol modified fibers can be characterized by the highest antioxidant activity, which can be associated to the acidic nature of the hydroxyl group [22].
As it was mentioned above, the different radical-scavenging effects observed can be attributed to the varying abilities of the individual phenolic compounds to react with DPPH, giving a stable non-radical product.
Using enzyme treatment, the surface of cellulose/chitin mix fibers is cleaned and etched and so more chitin is likely to be available at the surface to impart better antimicrobial properties to the fibers. Modification with phenolic antioxidants further improves the antimicrobial property. The antimicrobial activity was up to 100%.
The antibacterial effect of cellulose/chitin modified fibers was shown by the absence of growth after removing the fibres from the plate (Figure 6c). The absence of growth for modified fibers (after being re-incubated) shows that the new obtained fibers have an antibacterial effect (and not a bacteriostatic effect as in the case of growing bacteria). Absence of bacterial colonies under the specimen in the contact area is considered as an acceptable antibacterial activity.
The antimicrobial effect that phenolic compounds provided to cellulose/chitin mix fibers is in accordance with the results found out for the antimicrobial activity of unbleached kraft liner fibers [15]. The antibacterial mechanism of phenols is generally associated with the presence of hydroxyls and delocalisation of the electrons on their structure. Cellulase initiated modification of antibacterial aromatic structures increases the efficacy of these antibacterial substances and might be pursued as a valid methodology to build up covalently bound bio-active surfaces. Similar antimicrobial materials using cellulose/chitin mix fibers were obtained by plasma activation [7].
Cellulose/chitin mix fibers were successfully modified with p-hydroxybenzoic acid, gallic acid and eugenol using activation with a cellulase enzyme. The modification was evidenced using ATR-FTIR spectroscopy, X–ray photoelectron spectroscopy and scanning electron microscopy. The modification degrees estimated from the XPS data were 42.45% for CC/HBA, 36.92% for CC/ GA and 35.43% for CC/Eu.
Antioxidant capacity and microbiological activity of cellulose/chitin mix fibers modified with phenolic compounds were evaluated and the results showed that the efficiency depends on the type of phenolic compound used for modification. The antimicrobial activity and radical scavenging activity was increased up to 100%. Eugenol was found to impart the best antioxidant properties to cellulose/chitin mix fibers.
Antioxidant and antimicrobial properties of the modified cellulosic fibers recommend them as potential materials in the cosmeto-textiles field acting as a reservoir system capable of progressively deliver the active substances to the skin layers. Also, it is possible to be used as wound-dressing materials or as transdermal patches.
This modification procedure offer also the options of reinforcing hydrogels with solid supports and preparing membranes with potential applications in dialysis membranes, drug delivery carriers, etc.