ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Harsha Devnani1, Soami Piara Satsangee1 and Rajeev Jain2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
A novel sensitive electrochemical sensor has been developed by modification of glassy carbon electrode (GCE) with multi-walled carbon nanotube (MWCNT), chitosan (CHIT) and zirconium oxide (ZrO2) nanoparticles. The morphological characteristics of nanocomposite (MWCNT-CHIT-ZrO2 or MCZ) were studied by scanning electron microscope and atomic force microscopy. The electrochemical behavior of serotonin at nanocomposite modified GCE (MCZ/GCE) was investigated in pH 6.5 Briton Robinson buffer solution using cyclic voltammetry and square wave voltammetry. MCZ/GCE showed an enhancement in the current response as compared to bare GCE and showed a linear response to serotonin in the range 800 to 5000 nM. The limit of detection was found to be 0.6 nM, which is lower than many other sensors reported for serotonin in literature. The modified sensor showed high sensitivity (9 nA/nM) and selectivity for serotonin. The proposed method was successfully employed for quantification of serotonin in human blood serum samples.
Keywords |
| Serotonin, MWCNT, Chitosan, ZrO2, Glassy carbon electrode, Voltammetry |
INTRODUCTION |
| Serotonin (5-hydroxytryptamine, 5-HT) is an important catecholamine neurotransmitter which plays a vital role in the regulation of mood, sleep, emesis, sexuality and appetite. Of the total serotonin content, the central nervous system contains only less than 2% of it, but still plays an important role in various brain functions. It is synthesized from amino acid tryptophan. It acts as a pacemaker in various regions of the brain at times of alertness while co-ordinating sensory and motor activity. It also contributes to proper execution of feeding, sleeping and reproductive behaviours [1]. Fluctuations in the normal serotonin level are associated with a number of diseases. While low levels of 5-HT can cause depression, anxiety and migraines, high levels can result in toxicity and in extreme cases fatal effects such as serotonin syndrome, obsessive compulsive disorder, and autism [2-6]. |
| Thus, detection and determination of 5-HT are important and research thrust is to work out simple, sensitive, selective and fast analytical methods for its determination. Electrochemical method is given preference in this context over traditional methods due to their simplicity and sensitivity for determination of 5-HT [7]. Traditionally, neurotransmitterâÃâ¬ÃŸs concentrations are determined by chromatographic, spectrophotometry, fluorescence, chemical luminescence and capillary electrophoresis methods [8-11]. These techniques are complex, time-consuming, inconvenient for real-time and in-situ monitoring [12]. Since, 5-HT is electrochemically active it can be determined using electrochemical sensors which are fast and inexpensive. Some major problems are associated with the determination of 5-HT. Presence of dopamine (0.22 V) poses interference as it has oxidation potential close to that of 5-HT (0.38 V). Ascorbic acid and uric acid also have a similar oxidation potential and thus interfere with determination of 5-HT. Thus, the focus is on developing faster, cost-effective, sensitive and selective electrodes for the determination of 5-HT. Electroanalytical techniques fulfill these criteria but with bare electrodes they offer low sensitivity. Using modified electrodes, low sensitivity and poor selectivity can be overcome [5]. |
| The modification of electrodes with electron transfer mediators is gaining impetus in field of analytical chemistry. The importance of using any mediator is to cause an effect in reduction of the overpotential required for the electrochemical reaction, which results in sensitivity enhancement and an improvement of selectivity [13-14]. CNTs have attracted much attention in recent years in the field of electrochemical biosensing as they offer high effective surface area, mass transfer, catalysis and control over local microenvironment. CNTs are porous nanostructured carbon materials, which hold promise as immobilization substances due to their significant mechanical strength, excellent electrical conductivity and good chemical stability. A decrease in overpotential can be observed with CNTs owing to their catalytic property, producing a more reversible voltammetry than that displayed by same material in macroelectrode form [15,16]. Thus, CNTs can efficiently enhance electron transfer reactions. |
| Chitosan, a natural biobolymer obtained from chitin, possesses significant film-forming and adhesion properties. It has good biocompatibility and is non-toxic. Chitosan provides a hydrophilic environment to biomolecules as it has abundant amino and hydroxyl groups. Thus, it offers to improve hydrophilicity as well as biocompatibility via covalent grafting techniques. The co-existence of chitosan and CNTs creates a positive charge which is favorable for the further immobilization of biomolecules that are negatively charged [17-19]. |
| Further, metal nanoparticles can be used to enhance the amount of immobilized biomolecules in fabrication of a sensor. This capability is taken advantage of in biosensor design as metal nanoparticles have been used to catalyze biochemical reactions. In this context, zirconia has emerged as an important catalyst [20,21]. In the present communication, we have fabricated a nanocomposite comprising MWCNT, Chitosan and ZrO2 for the sensitive and selective determination of 5- HT. Electrochemical analysis was carried out under the optimized conditions using cyclic voltammetry (CV) and square wave voltammetry (SWV) in BR buffer; pH 6.5. MCZ/GCE showed a remarkable enhancement in the current response of oxidation process of 5-HT in comparison to the bare GCE which was described on the basis of the interlayer mass transport regime within the porous layer of MCZ [22-25]. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) were employed for the structural and morphological analysis of the nanocomposite. Electrochemical response characteristics of 5-HT at MCZ/GCE were found to be a function of pH, scan rate and the concentration. The fabricated sensor was further successfully applied for the estimation of 5-HT in the human blood serum. |
MATERIALS AND METHODS |
Instrumentation: |
| All electrochemical studies were performed at a PC-controlled AUTOLAB PGSTAT 302N (Eco-Chemie B.V., Utrecht, The Netherlands) potentiostat-galvanostat with IME663 and software NOVA 1.8. EIS was carried out using FRA 2 module. A standard three electrode electrochemical assembly was used in the study that contained GCE and MCZ/GCE as working electrode, platinium wire as counter and Ag̢̉̉AgCl (3M KCl) as reference electrode that were fitted in one compartment cell connected with electrochemical workstation through Metrohm 663VA stand. The electrochemical cell was fitted with the nitrogen gas bubbler. All pH measurements were made on a Mettler Toledo pH meter fitted with a glass electrode and a Ag̢̉̉AgCl electrode as reference which was pre-standardized with buffers of known pH. All measurements were carried out at room temperature. A DL-180 ultrasonic apparatus was used for sonicating the suspension of MWCNT, MWCNT-CHIT and MCZ for modifying GCE. AFM study was carried out at Nanosurf Easyscan (Switzerland) with software Nanosurf 1.8. SEM was performed at Tescan (7718) involving the software, Mira TC. |
Materials and Reagents: |
| ZrO2 (<100 nm) was procured from Sigma Aldrich (India) and Chitosan was purchased from MP Biomedicals (India). MWCNT (10 nm) was procured from Drop Sens. Serotonin standard (≥99%) was obtained from the Sigma Aldrich. Ultra pure water (Milli-Q water with resistivity 18MΩ.cm) was obtained from ELGA purification system (U.K). Standard solution of 5-HT (10 mM) was prepared by dissolving pure compound in 0.1 M perchloric acid and was further diluted with phosphate buffer to get the concentration in the working range. Solutions at all the stages of the study were prepared by using analytical grade reagents and were used without further purification. |
Fabrication of MWCNT, MWCNT-CHIT and MCZ modified sensor: |
| Prior to modification, the bare working electrode (GCE) was polished against the alumina slurry (particle size- 0.05−0.3μm) spread over the Buehler cloth, followed by gently blowing under the nitrogen stream to remove the residual alumina particles and then sequentially rinsed with de-ionized water. For modification, MWCNT-CHIT was obtained by dispersing 0.5 mg of MWCNT in 1mL of 0.5 (wt) % of CHIT solution (in DMF) and another suspension of MCZ was obtained by dispersing 0.5 mg of ZrO2 in 1mL of the suspension containing 0.5 mg of MWCNT and 0.5 (wt) % of CHIT solution with ultrasonication for 5 hours. MWCNT (0.5 mg in DMF) alone was also used as a modifier. 1 μL of each of the modifiers was withdrawn and drop casted over the surface of GCE to get MWCNT/GCE, MWCNT-CHIT/GCE and MCZ/GCE. The modified electrodes were kept for drying at room temperature. |
Analytical Procedure: |
| The stock solution of 5-HT (10 mM) was prepared in 0.1 M HClO4. Working solutions were prepared by further dilution with the supporting electrolyte to get the desired concentration range. Initially, a series of BR buffer (2.5-12 pH) was prepared in ultrapure water and used as supporting electrolytes. About 10 mL of electrolyte solution containing appropriate amount of standard 5-HT or sample were added to the electrolytic cell. Prior to all the electrochemical measurements, all the solutions were purged with the pure N2 gas to remove the interference due to O2. Then the electrodes were immersed and the SWV and CV were recorded with potential between -0.2 to 0.8 V. The surface of GCE was renewed prior to the modification by abrading the surface smoothly against the alumina slurry spread over the Buehler cloth. All voltammetric measurements were carried out at ambient temperature. |
RESULTS AND DISCUSSION |
Characterization of the modifiers: |
| The surface morphology of the modifiers was studied using SEM technique. The modifier suspension was drop-casted over the surface of ITO (Indium Tin Oxide) section for carrying out analysis. The SEM images of MWCNT (A) MWCNT-CHIT (B) and MCZ (C) are depicted in Fig. 1. The MWCNT and MWCNT-CHIT modifiers show a rough surface owing to random dispersion of MWCNT in the within the chitosan matrix as well as the solvent (DMF). The MCZ nanocomposite SEM image (C) exhibits a different surface morphology due to the presence of globular ZrO2 nanoparticles with porous topology within the chitosan and graphene matrix. This is outcome of the electrostatic interactions between the cationic chitosan and the surface charged ZrO2 nanoparticles [26]. The formation of a codispersion of MWCNT, CHIT and ZrO2 resulting in a nanocomposite is thus explained. |
 |
| The surface topology which is indication of roughness parameters of the electrode surface was interpreted using AFM. The surface area was found to be 26.8 pm; roughness average, 123.7 nm; root mean square, 150.2 nm; peak-valley height, 960.45 nm; peak height, 377.35 nm; valley depth, 529.1 nm and mean value to be 120.53 pm The data implies that the modifier is well-coated onto the surface of GCE. The nanocomposite coatings were not removed from the surface of GCE by several flushings with deionized water (Fig. 2). |
 |
Electrochemical characterization of modified electrodes: |
| The electrochemical response of K3Fe(CN)6 at bare and modified GCE (Fig. 3) was also checked to examine their electrocatalytic properties. In comparison to bare GCE, better electrochemical response was observed for K3Fe(CN)6 at modified electrodes. The maximum enhancement was seen in case of MCZ/GCE with decreasing over potential. The peak separation was seen to be considerably reduced at the MCZ/GCE. At bare GCE the anodic peak potential (Epa) was found to be 266 mV and cathodic peak potential (Epc) 149 mV. The separation of redox potential peaks (Ep) 111 mV and the ratio of peak current (Ipa/Ipc) was 1.06. At MCZ/GCE, a pair of redox peak is obtained with strong increase in both anodic and cathodic peak current. The Epa was found at 239 mV and Epc at 81 mV. The separation of redox potential peaks Ep was found to be 117 mV and the Ipa/Ipc was 1.02. |
 |
| Fig. 3. Cyclic voltammetric response of the standard redox system (K3Fe(CN)6) (1 mM) at GCE (a), MWCNT/GCE (b), MWCNT-CHIT/GCE (c) and MCZ/GCE (d) |
| The electro-active area of the MWCNT/GCE, MWCNT-CHIT/GCE, MCZ/GCE and bare GCE were obtained by the CV using 1 mM K3 Fe(CN) 6 as a redox indicator at different scan rate. The peak current Ip can be calculated using Randles Sevcik equation at 25 ºC [27]: |
 |
Electrochemical behavior of 5-HT at the modified electrodes: |
| The electrochemical determination of 5-HT was analyzed at bare GCE and at the modified electrodes using CV and SWV techniques. Fig. 4A and 4B respectively represent the CV and SWV of 5-HT (1 μM) at bare GCE (a), MWCNT/GCE (b), MWCNT-CHIT/GCE (c) and MCZ/GCE (d). |
| A well-defined oxidation peak is observed at bare GCE and also at each of the modified electrodes that can be ascribed to the oxidation of 5-HT to its respective quinine (serotonin quinoneimine) [28,29]. While at MWCNT/GCE and MWCNT-CHIT/GCE the current response is enhanced but the base line current is also considerably raised. At MCZ/GCE, maximum current enhancement is observed and base line current was also not raised much. The enhancement in current response is attributed to increase in the surface area owing to porosity of the modified layer. The enhanced anodic peak current intensity is due to the trapping of the solution containing 5-HT within the multiple layer of the porous nanocomposite [22,25]. Thus, MCZ/GCE proved to be highly sensitive towards the oxidation of 5- HT and thereby it was applied for rest of the electrochemical measurements. |
 |
Effect of supporting electrolyte and pH: |
| The nature of electrolyte and pH of the medium affect the peak shape, height and stability of voltammograms (SWV). The influence of various supporting electrolytes such as KCl, phosphate buffer, acetate buffer and Britton Robison (BR) buffer on the electro-oxidation of 5-HT (1 μM) was investigated to optimize the experimental condition. BR buffer (0.1 M) gave maximum current response accompanied with optimum peak shape and stability. The influence of pH on the 5-HT oxidation (1 μM) at MCZ/GCE was examined over the pH range 2.5−9 by SWV. The anodic peak current intensity was found to be sensitive towards the pH and it gave a maximum response at pH 6.5. The sensor is thus suited for physiological applications as it shows best response in pH 6.5 while losing its sensitivity in both highly acidic and basic conditions. On the basis of these results phosphate buffer at pH 6.5 was selected as the optimum supporting electrolyte. A sequential shift of the anodic peak potential with the augmentation of solution pH was also observed (Fig. 5), indicating that the protons participate in the electrode reaction process of 5-HT [30,31]. A linear dependence of peak potential on pH of the supporting electrolyte was obtained following a linear equation: E/mV = 787.4 – 62.64 pH; R2 = 0.997. The peak potential at 25 ºC is expressed as [32]: |
 |
 |
Effect of scan rates: |
| The influence of varying scan rates (40-180 mV/sec) on the electro-oxidation process of 5-HT (1 μM) at MCZ/GCE is shown in Fig. 7. The anodic peak current intensity was found to increase with increasing scan rate. A linear plot of peak current intensity (Ip) vs. square root of scan rate (ν) was obtained (Fig. 6), following the linear equation: Ip (μA) = 0.0524 ν (mV/s) - 0.2488; R2 = 0.99, which implies that the redox process of 5-HT at the MCZ/GCE is diffusioncontrolled [33]. Also, the difference between the anodic peak potential and the cathodic peak potential, ΔEp was observed to increase with increasing scan rate. No reverse peak was observed at lower scan rates while relatively small cathodic peaks were observed at higher scan rates which implies irreversible or quasi-reversible electrode process for 5- HT. |
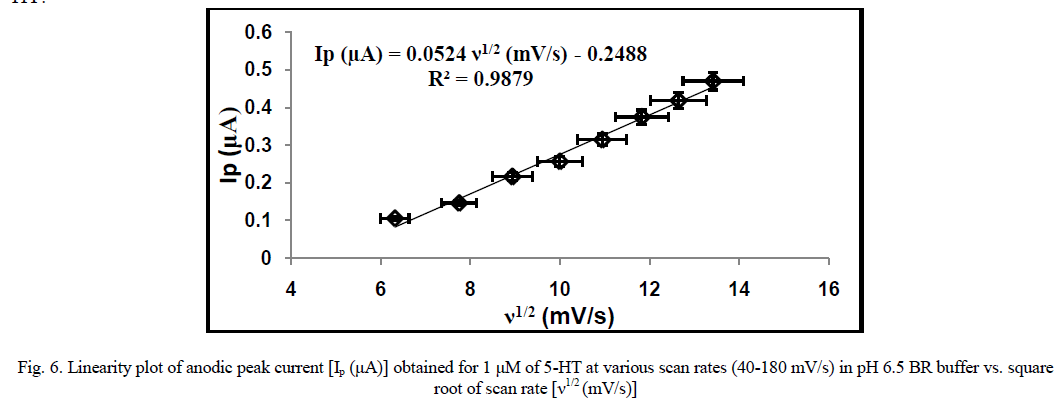 |
Determination of electrode dynamics parameters (α and n) and proposed mechanism of 5-HT: |
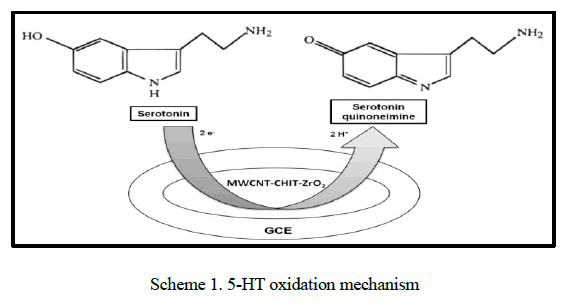
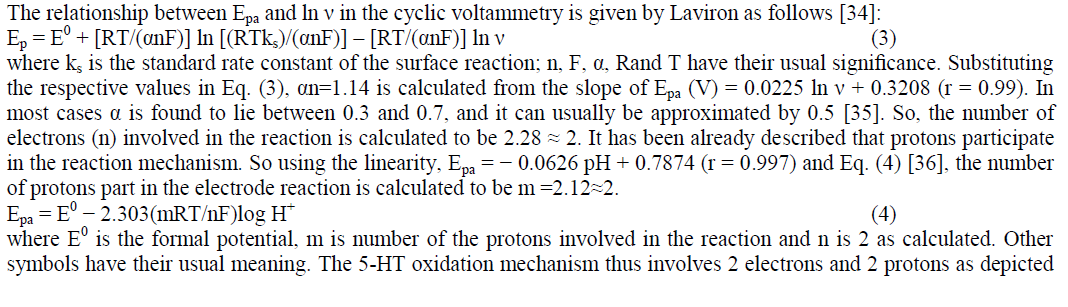 |
| in scheme 1. The electrochemical oxidation of 5-HT is believed to occur at the phenol group in the molecule to form the corresponding ketone. The absence of the corresponding reverse peak indicates the instability of the oxidation product, which then undergoes chemical reaction to form a product that is easily oxidizable. [28, 29]. |
 |
Calibration curve, detection limit and method validation: |
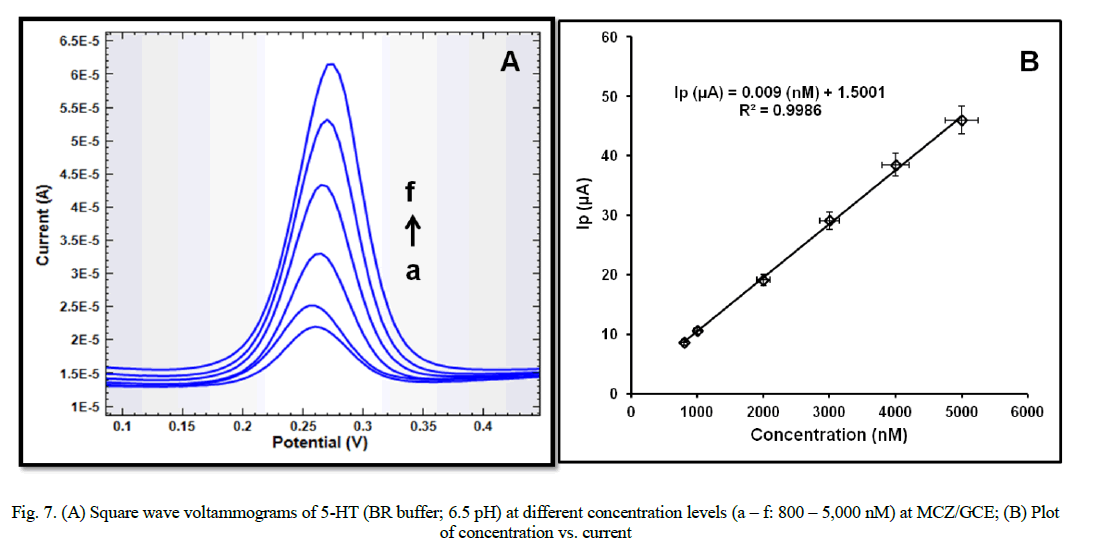
| SWV recorded with the increasing amounts of 5-HT under the optimized conditions exhibited linearity of the peak currents with the increasing concentration (Fig. 7A). A linear relationship between the peak current and the 5-HT concentration was observed in the range of 800 to 5,000 nM. The calibration plot of I (μA) vs. concentration (nM) (Fig. 7B) is described by the equation:- |
| I (μA) = 0.009 (nM) + 1.5001; R2 = 0.9986 |
| Limit of detection (LOD) and limit of quantification (LOQ) (estimated as 3 S/m and 10 S/m, respectively, „SâÃâ¬ÃŸ being the standard deviation and „mâÃâ¬ÃŸ being the slope of the calibration curve) were obtained as 0.6 nM and 2 nM. Various statistical parameters for the linear regression equation have been evaluated and reported in table 1. |
 |
 |
Reproducibility and stability of fabricated sensor: |
| The reproducibility of the modified electrode was investigated by the successive measurement of 5-HT (1 μM) at MCZ/GCE by SWV (N = 6). The corresponding relative standard deviation of 1.7 % showed that the results are satisfactorily reproducible. |
| Another important attraction of the proposed modified electrode is that the resulting modified electrode is of long term stability. Stability of the proposed modified electrode was tested by measuring the decrease in voltammetric current during repetitive SWV measurements of 5-HT (1 μM) with MCZ/GCE stored in air over a period of two weeks. After two weeks, it was found that the corresponding current response fell less than 2 % and showed a RSD of 1.94 %. The reproducibility results are reported in table 2. |
 |
Interference Study: |
| The influence of various compounds that are expected potentially to interfere with the determination of 5-HT (1 μM) were studied under the optimum conditions in pH 6.5 BR buffer. The tolerance limit was defined as the maximum concentration of the interfering substance that caused an error of less than ±5 % for the determination of 5-HT. The interfering species were chosen from substances commonly found with 5-HT in pharmaceutical formulations and/or in biological fluids. In biological environments, the main interference is the presence of high concentration of ascorbic acid and uric acid. While ascorbic acid didnâÃâ¬ÃŸt pose any interference up to 150 folds, uric acid was tolerated up to 300 folds. Dopamine also interferes with determination of 5-HT as they have close oxidation potentials. At MCZ/GCE, peak separation of 82 mV among these interferents was observed and thus allowing reliable and selective determination of 5-HT. The results depicted the high selectivity of the MCZ/GCE for the determination of 5-HT. |
Assay of 5-HT in human blood serum: |
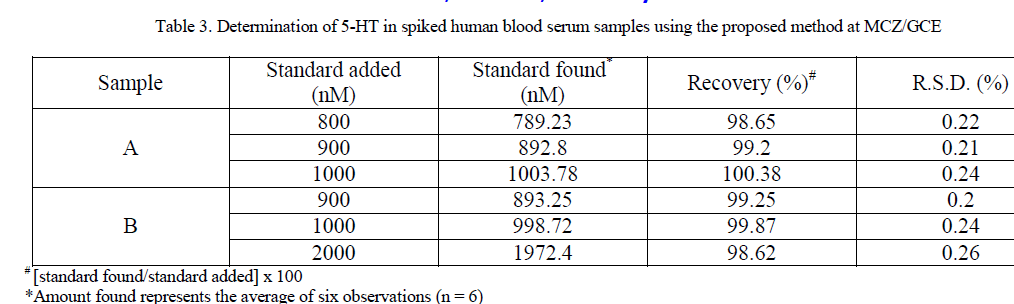
| For the purpose of the practical applicability of the proposed method and the modified sensor, determination of 5-HT in human blood serum were performed by spiking diluted samples with known amounts of 5-HT. The human blood serum was obtained from a blood bank. To evaluate the accuracy of the method, standard addition method was used by spiking different concentration of 5-HT in the sample solution. SWV of unspiked and spiked samples was performed under optimized conditions at MCZ/GCE. Percentage recoveries have been reported in table 3. The good recoveries confirm that the detection of 5-HT at the modified sensor, MCZ/GCE is a reliable method for direct determination of 5- HT in human blood samples. |
 |
CONCLUSION |
| A novel MWCNT-CHIT-ZrO2 nanocomposite modified sensor was fabricated to study the voltammetric behavior of 5- HT. The resulting MCZ/GCE sensor exhibited higher sensitivity to serotonin as compared to the MWCNT/GCE, MWCNT-CHIT/GCE and bare GCE due to the synergistic effect of MWCNT, CHIT and ZrO2 nanoparticles facilitating the electron transfer process. The surface modifier was successfully characterized by SEM and AFM. Effect of pH of the supporting electrolyte and varying scan rates on the response characteristics of 5-HT exhibited that its electro-oxidation process at MCZ/GCE is a proton coupled and diffusion controlled process. Ascorbic acid and uric acid were found not to pose interference to quite a good extent. The practicable applicability of the proposed method with higher sensitivity, specificity, reproducibility and accuracy was applied for the estimation of 5-HT in human blood samples. |
ACKNOWLEDGEMENTS |
| The authors greatly acknowledge their work to the Ministry of Human Resource and Development (MHRD), India, for providing funds for carrying out the research work and DST INSPIRE. They are also thankful to Dr. D. K. Avasthi, Dr. S. A. Khan and Mr. Sunil for providing lab facility for carrying out SEM at Inter University Acceleration Centre, New Delhi, India. |
References |
|