ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Teena Thomas1, Ayswarya E. P. 2 and Eby Thomas Thachil3 Research Scholar, Dept. of Polymer Science & Rubber Technology, Cochin University of Science & Technology, Cochin 22, Kerala, India1 Research Scholar, Dept. of Polymer Science & Rubber Technology, Cochin University of Science & Technology, Cochin 22, Kerala, India2 Professor (Rtd), Dept. of Polymer Science & Rubber Technology, Cochin University of Science & Technology, Cochin 22, Kerala, India3 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Nano alumina (Al2O3) was prepared by gel combustion method. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to characterize the nano alumina. The results show that the prepared alumina was in nano meter range. It was added as reinforcing filler in natural rubber. Mechanical property improvements were achieved by the addition of low amounts of nano alumina along with maleic anhydride as compatabilizer.
Keywords |
| nano alumina, natural rubber, reinforcement, mechanical properties |
INTRODUCTION |
| Polymers are a versatile class of engineering materials because of their ability to be tailor-made to suit specific requirements. Fillers play a dominant role in modifying the properties of the base polymer. In rubber industry, a variety of fillers are used to improve and modify the physical properties of elastomeric materials. The addition of rigid filler particles, even in small amounts, to an elastomer, strongly influences its response to mechanical stimuli. Nano fillers are the ultimate choice in fillers because of their ability to modify properties at very low levels of incorporation. In recent years there has been an increasing interest in the use of nano metal oxides as reinforcing fillers [1]. Alumina is one of the important ceramic materials. Nano crystalline Al2O3 powder has considerable potential for a wide range of applications including high strength materials, electronic ceramics and catalysts. In this work, we try to exploit the reinforcing nature of nanoalumina powder in natural rubber [2]. In order to obtain better interaction between the matrix and filler an adhesion promotion mechanism using maleic anhydride grafting was also tried. Maleic anhydride grafted onto the double bond in the natural rubber at cure temperature will impart polarity to the natural rubber. This will improve the interaction of polar alumina with natural rubber thereby increasing the reinforcement [3, 4]. Nanoalumina was prepared by the gel combustion method starting from aluminium nitrate. This is a simple technique employing only a low temperature [5]. Nevertheless this is probably the first attempt to prepare nanoalumina using gelatin as the gelling medium and subsequent application in rubber processing. |
II. MATERIALS AND METHODS |
| Crystalline aluminum nitrate (AR), gelatin (Chemical grade) and maleic anhydride (AR) were supplied by Merck India Limited. Natural rubber (ISNR-5) was obtained from the Rubber Research Institute of India, Kottayam. Zinc oxide, stearic acid, CBS (N-cyclohexylbenzothiazole-2-sulphamide), TMTD (tetramethyl thiuram disulfide) and sulfur used were of commercial grade. |
| A. Preparation of Nano Alumina |
| Gelatin was first dissolved in hot distilled water. Aluminum nitrate was also dissolved in distilled water and added drop wise to the gelatin solution with stirring. After the completion of addition, the resultant solution was concentrated to a gel and incinerated in a muffle furnace at 900âÃÂðC for two hours. |
| Phase identification of the nanoalumina was carried out by XRD using a Bruker, D8 Advanced Model employing CuKα. The shape and morphology of the particles were analyzed by scanning electron microscopy (SEM) Joel Model JSM 6390 LV. |
| B. Preparation of Nano Composites |
| Formulations of the composites used in the study is shown in Table 1. In the first set of composites, content of alumina was varied as 0, 0.5, 1, 1.5 and 2 phr. In the second set, one phr maleic anhydride wasadded along with different concentrations of alumina. All other ingredients were kept at the same level. |
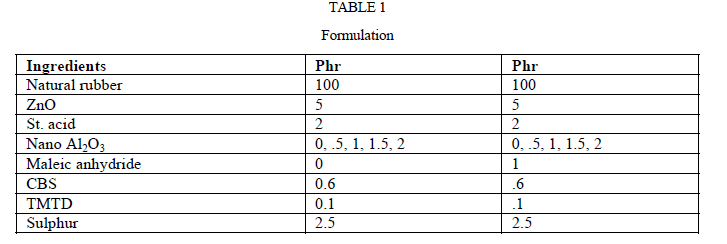 |
| Mixing and homogenization of rubber and compounding ingredients were done on a laboratory size (150 × 300 mm2) two-roll mill at a friction ratio of 1:1.25. Once a smooth band of rubber was formed on the front roll, compounding ingredients were added as per ASTM D 3184 (1980) in the order, activators, filler, accelerators and curing agent. After complete mixing the stock was passed six times through tight nip and finally sheeted out at a fixed tight nip gap. Cure characteristics of the mix were determined as per ASTM D 2084-1995 using Rubber Process Analyzer, RPA 2000. The test samples were vulcanized in standard moulds on an electrically heated press having 30×30 cm2 platens at a fixed pressure of 200 kg/cm2 to their respective cure times. The samples obtained were tested for mechanical properties according to relevant ASTM standards. |
III. RESULTS AND DISCUSSION |
| A. X-ray Diffraction (XRD) |
| X-ray diffraction pattern of the nanoalumina is shown in Figure 1. Peaks corresponding to the 2θ values of 32, 47 and 68 degrees indicate that γ-phase is prominent among the phases of the prepared nano Al2O3 [6]. |
 |
| B. SEM |
| Scanning electron microscopic image of the prepared alumina is shown in figure 2. From SEM photograph it is clear that the prepared Al2O3 particles are in nano size, typically 50 to 90 nm. Agglomerates of the particles were also observed. |
 |
| C. Cure Characteristics |
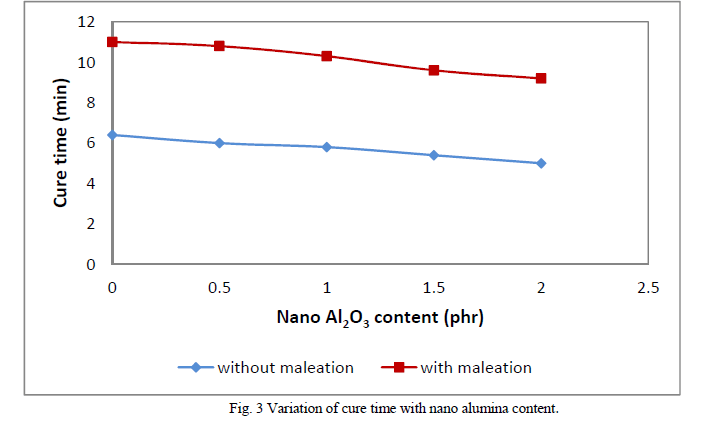
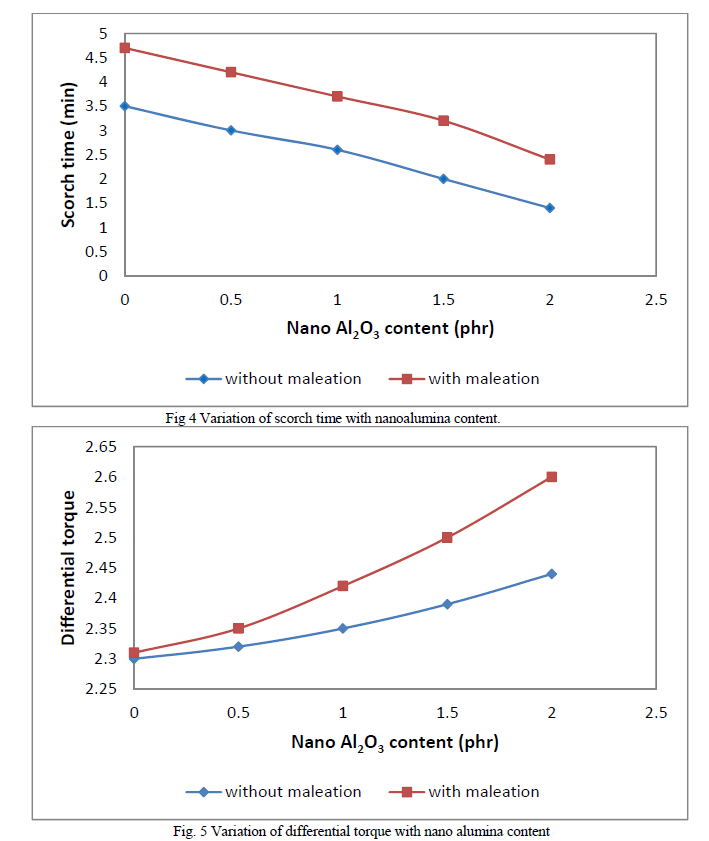
| Figures 3 and 4 show variation of cure time and scorch time respectively with filler (nanoalumina) content. Compared with non maleated samples maleated samples show higher cure time. This may be due to the acidic nature of maleic anhydride which retards the cure. But, in both sets of composites, with increase in filler loading, cure time decreases. This shows the accelerating effect of nanoalumina on rubber curing. Scorch time also follows the same trend. The influence of nanoalumina on rubber crosslinking may be explained by the basic nature of alumina which causes the acceleration of cure rate. Figure 5 shows the variation of differential torque with filler concentration. Differential torque increases with filler loading indicating an increase in the modulus of the composite. |
 |
 |
| D. Mechanical Properties |
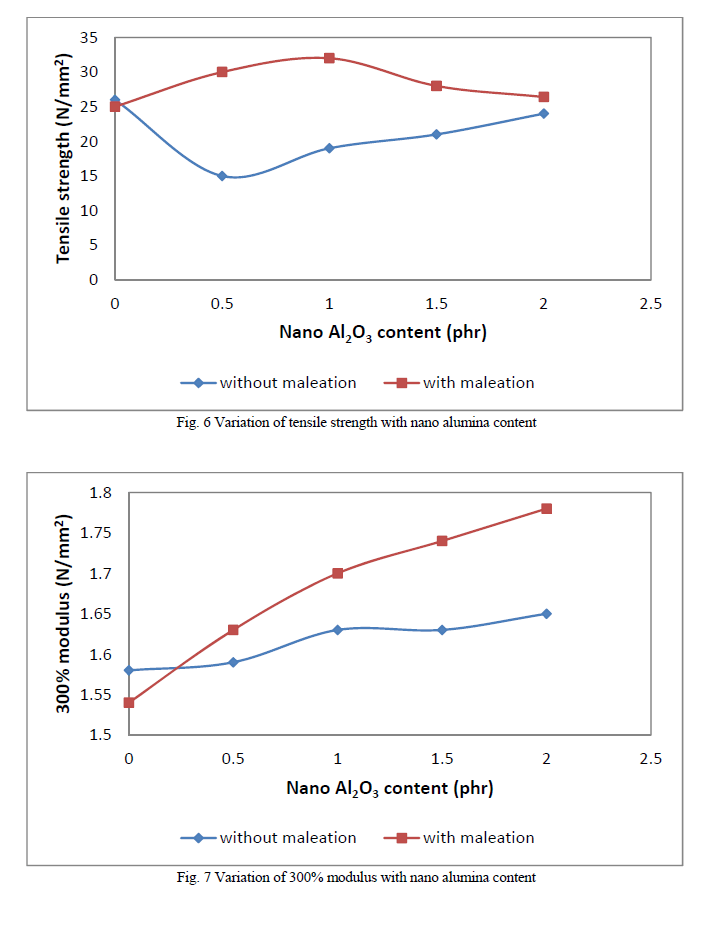
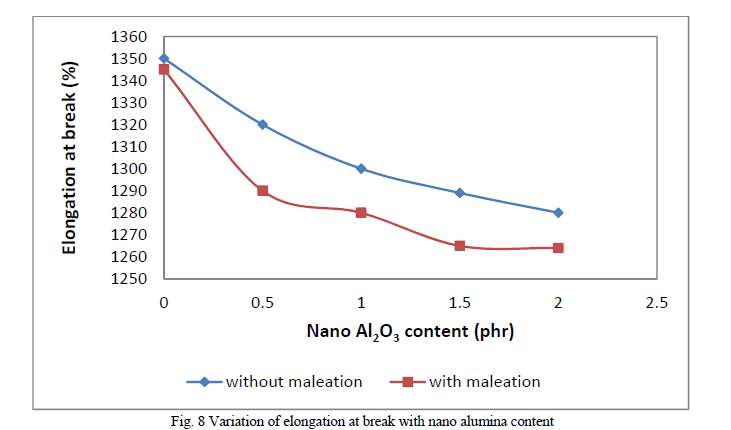
| Figure 6 shows the variation in tensile strength with nano alumina loading. Tensile strength shows a decrease with filler loading in the case of non maleated composites. This may be due to the disruption of the inherent strain-induced crystallization of the natural rubber by the filler particles. Upon maleation the matrix-filler interaction increases and hence tensile strength also increases. But the reinforcing effect is more pronounced at lower filler loading. This may be due to the agglomeration of filler particles occuring at higher percentages of filler loadings. These agglomerates will act as points of weakness causing the premature failure of the composites. Figure 7 shows the variation of modulus with filler loading. Modulus shows a linear increase with the filler loading in both cases. At low strains where modulus measurement of the composites were carried out, agglomeration of filler particles does not have a detrimental effect. Variation of elongation-at-break is shown in Figure 8. Elongation-at-break decreases with filler loading in both cases of non maleated and maleated composites. |
 |
 |
IV. CONCLUSION |
| The sol-gel method was successfully used for the preparation of nanoalumina. XRD spectrum of prepared Al2O3 shows that the nanoalumina formed is in the γ- phase. Prepared Al2O3 particles are in the nanometer range as evident from SEM analysis. The incorporation of nano Al2O3 into natural rubber accelerated the curing process and improved curing characteristics. Mechanical properties of the composites, especially tensile strength, were enhanced by the incorporation of nano alumina. |
References |
|