e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Pharmaceutical Chemistry, University College of Pharmacy, Regional Institute of Medical Science and Research, Mahatma Gandhi University, Kottayam, Kerala, India.
2Department of Pharmaceutical Sciences, Cheruvandoor, Mahatma Gandhi University, Kottayam, Kerala, India.
Received date: 22/08/2014; Revised date: 16/09/2014; Accepted date: 22/09/2014
Visit for more related articles at Research & Reviews: Journal of Chemistry
This research was focused on the rational approach in design and development of novel azetidinone derivatives comprising 1, 2, 4-triazole for anti-tubercular activity. The present work involved the preliminary in silico designing of the various novel azetidinone analogues for quantifying their drug likeness. Molecular docking experiments were carried out to identify potential drug candidates among azetidinone analogues. A new series of 1-(substituted phenyl)-4-(substituted phenyl)-3-(4H-1, 2, 4-triazol-4-yl amino) azetidin-2-ones were synthesized and evaluated for their anti tubercular activity. Different azetidinones were prepared by reacting Schiff bases with chloroacetyl chloride in the presence of catalytic amount of triethylamine. The subsequent amino azetidinones were synthesized using 4-amino 1, 2, 4-triazole. All the synthesized compounds were characterized by elemental analysis, IR, 1H NMR and mass spectral studies.3-nitro and 4-methoxy benzaldehyde analogues of azetidinone derivatives showed good anti-tubercular activity against Myco- bacterium tuberculosis H37RV strain by Alamar Blue assay method compared to standard drugs.
Schiff bases; 1, 2, 4-triazole; Azetidinone; Antitubercular.
Tuberculosis (TB) is a chronic infectious disease caused by mycobacteria of the ‘‘tuberculosis complex’’, which includes primarily Mycobacterium tuberculosis in humans. The cause of TB, M. tuberculosis, is a small aerobic non-motile bacillus. High lipid content of this pathogen accounts for many of its unique clinical characteristics. Other TB-causing mycobacteria include M. bovis, M. africanum, M. canettiand, M. microti. It is a respiratory transmitted disease affecting nearly 32%of the world’s population, more than any other infectious disease [1].
Tuberculosis (TB) remains as a major global health problem. It causes ill-health among millions of people each year and ranks as the second leading cause of death from an infectious disease worldwide, after the human immune deficiency virus (HIV). The latest estimates included in WHO’s report are; there were almost 9 million new cases and 1.4 million TB deaths in 2012(990 000 among HIV negative people and 430 000 HIV-associated TB deaths). The provision of diagnosis and treatment according to the DOTS/Stop TB Strategy has resulted in major achievements in TB care and control.
There are more than twenty drugs that are currently used for the treatment of TB and almost all of them were developed some years ago. The drugs are used in differing combinations in different circumstances, so that for example some TB drugs are only used for the treatment of new patients who are very unlikely to have resistance to any of the TB drugs.
The basic TB drugs (1st line drugs) are; Ethambutol, Isoniazid (INH), Pyrazinamide, Rifampicin, Streptomycin. All the other TB drugs are generally referred to as “second line” or reverse TB drugs.
Computational chemistry and molecular modelling have become an essential part and an emerging tool for research and drug development process. The process of drug discovery is very complex and requires an interdisciplinary effort to design effective and commercially feasible drugs. The objective of drug design is to find a chemical compound that can fit to a specific cavity on a protein target both geometrically and chemically [2].
Molecular docking
Molecular docking is a computer simulation procedure to predict theconformation of a receptor-ligand complex, where the receptor is usually a protein ora nucleic acid molecule (DNA or RNA) and the ligand is either a small molecule oranother protein [3]. It can also be defined as a simulation process where a ligand position is estimated in a predicted or pre-defined binding site. The aim is to achieve anoptimized conformation for both the protein and the ligand and relative orientation between them such that the free energy of the overall system is minimized. The two main stages of molecule docking are: a search stage that configures a certain numberof possible binding of given protein and ligand and a scoring stage that estimates binding affinity for given protein, ligand, pose and conformations [4].
Lead compound
The objective of this drug discovery phase is to synthesize lead compounds. A lead compound in drug discovery is a chemical compound that has pharmacological or biological activity and whose chemical structure is used as astarting point for chemical modifications in order to improve potency, selectivity, or pharmacokinetic parameters.
Heterocyclic compounds as lead
Heterocyclic compounds have a wide range of applications. Their applications in pharmaceutics are because of their specific chemical reactivity. They are very widely distributed in nature and are very essential to living organisms. They play a vital role in the metabolism of all the living cells[5].
Among large number of heterocycles found in nature, nitrogen heterocycles are the most abundant specially those containing oxygen or sulphur due to their wide distribution in nucleic acid illustration and their involvement in almost every physiological process of plants and animals. Almost 80% of the drugs in clinical use are based on heterocyclic constitution because they have specific chemical reactivity. Majority of the large number of drugs being introduced in pharmacopoeias in recent year are heterocyclic compounds. Various studies showed that heterocyclic ring with electron donating group increases the anti-TB activity [6].
Azetidinone as lead
Azetidin-2-ones, commonly known as β-lactams, are well-known heterocyclic compounds among the organic and medicinal chemist [7]. Azetidin-2-ones are 2-carbonyl derivatives of the 4-membered heterocyclic compounds containing nitrogen atom in the ring [8].The β-lactams are 4-membered cyclic amides derived from 3-aminopropanoic acids. The β-lactams as a class acquired importance since the discovery of penicillin which contains β-lactam unit as an essential structural feature of its molecule [9].A lot of research work on azetidinones has been done in the past. The nucleus is also known as wonder nucleus because it gives out different derivatives with all different types of biological activities.
Triazole as lead
Triazoles are 5-membered rings, which contain two carbon and three nitrogen atoms. According to the position of nitrogen atoms the triazoles are exists in two isomeric forms; the 1,2,3-(1,2,5) and the 1,2,4-(1,3,4)-, the former being known as osotriazole, and the latter as triazole [10].Triazoles and its derivatives possess a great importance in medicinal chemistry and can be used for the synthesis of numerous heterocyclic compounds with different biological activities that include anti tubercular [11,12], antidepressant activity [13], anti-convulsant activity [14], anti-cancer [15] and antimicrobial properties [16,17].
Materials
In silicomolecular modelling studies were carried out on various softwares like Schrodinger, ACDLABS ChemSketch and Molinspiration.
The chemicals and reagents used in the present work were of AR and LR grade, procured from Merck, Spectrum, Hi-Media, Nice and Sigma-Aldrich. All the chemicals were dried and purified wherever necessary. The melting points of the synthesized compounds were determined by Thiels melting point apparatus (open capillary tube method) and all the compounds gave sharp melting points and were uncorrected. Purity of the compounds was ascertained by thin layer chromatography. The IR spectra of the synthesized compounds were recorded on IR affinity-1 FTIR spectrophotometer Shimadzu in the range of 400-4000.The NMR Spectra of the characteristic compound was recorded by NMR 400 MHZ Spectrophotometer Brucker. The mass spectrum of the characteristic compound was recorded by JOEL GC mass spectrometer using electron ionisation method.
In silico design procedure
The 3-D structure of the protein was obtained from PDB using their specific PDB ID (4DRE). The protein structure was prepared using the protein preparation wizard in the Schrodinger software graphical user interface Maestro v9.3. A set of 1,2,4-triazole incorporated azetidinone analogues were selected as ligands and their structures were drawn using the workspace of Maestro and were converted to 3D form for the docking studies. The collected ligands were prepared for docking. Then the prepared ligands were docked into the generated grid in the prepared protein. The best docked pose with lowest Glide score value was recorded for each ligand. Extra precision (XP) was performed using the module Induced Fit Docking of Schrödinger-Maestro v9.3 (2012). Best derivatives with good docking score were selected and their ADME properties were checked using QIKPROP which is a tool available in Schrodinger under Maestro. The Lipinski’s rule of five and drug likeness analysis of selected derivatives are also calculated [18].
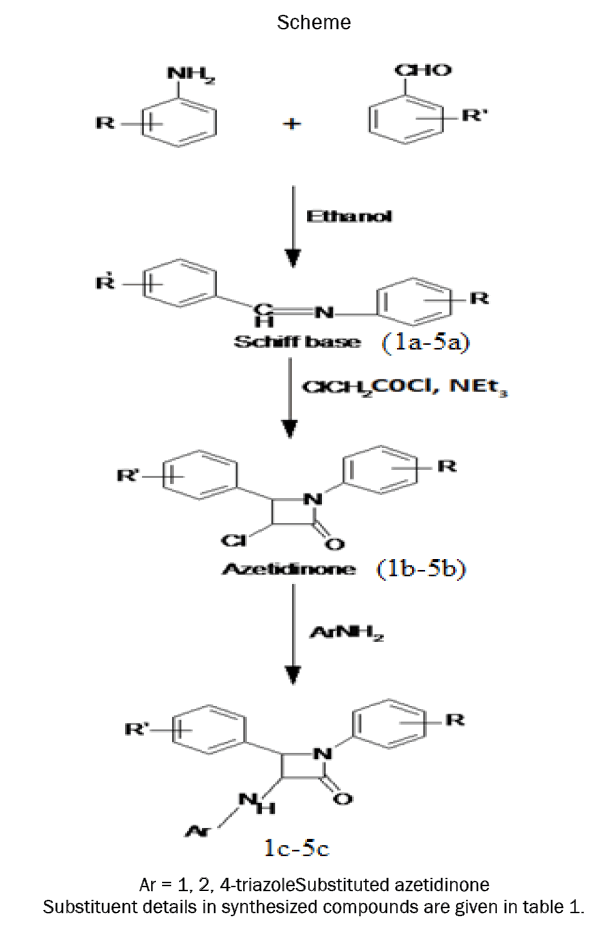
Synthesis of Schiff base
Equimolar quantities of substituted benzaldehydes and substituted anilines dissolved in ethanol are taken in a round bottom flask. To this added 2 drops of glacial acetic acid and was refluxed for 3 hours and the completion of reaction monitered by TLC. Then the reaction mixture was poured into crushed ice. The precipitate obtained was filtered and washed with cold water to obtain compound 1a-5a. Dried product was recrystallized from ethanol [19]. Physicochemical properties of different Schiff bases are given in table 2.
Synthesis of azetidinones
0.01 mol of Schiff base (1a-5a) and triethylamine (10 mL) in dry 1,4-dioxane (10 mL) was stirred well at 0°C-5°C temperature. To this mixture chloroacetyl chloride (0.01mol) was added drop wise for half an hour. The mixture was then shaken by a flask shaker for additional 5 hours and left 48 hours at room temperature. The mixture was concentrated, cooled, poured into ice cold water. The product obtained was filtered, washed with cold water and then dried [20]. The solid product was recrystallized from ethanolto obtain compound 1b-5b. Physicochemical properties of different azetidinones are given in table 3.
Synthesis of substituted azetidinones
Azetidinones (0.005mol) and 4-amino-1, 2, 4-triazole (0.005mol) were separately dissolved in 1,4-dioxane and mixed in an RBF. Then triethylamine (0.005mol) was added and the reaction mixture was refluxed for 4 hours. The reaction was monitoredby TLC. The reaction mixture was then dumped in ice cold water andthe precipitate was collected by suction and dried. The products (1c-5c) were recrystallised from acetone [21].Physicochemical properties of different amino azetidinone derivatives are given in table 4.
The current research revealed the significance of rational designing for the development of novel 2-azetidinone derivatives. The newly synthesized derivatives were anticipated as promising leads having many biological activities. Keeping this view in mind, the new analogues were designed, synthesized and evaluated for antitubercular activity.
Docking result
Glide scores of designed analogues of substituted 2-azetidinones and corresponding 2-azetidinones are given in table 5 and docking image of Mycobacterium tuberculosis protein enoyl ACP reductase is given in figure 1 [22].
Lipinski's rule of the synthesized compound [23]
All the synthesised derivatives obey Lipinski’s rule of five and the values are given in table 6.
Drug likeness analysis [24]
Drug likeness analysis parameters of selected derivatives for synthesis were compared to standard anti TB drugs and are given in tables 7 and 8.
Characterization
4-(2-chlorophenyl)-1-(4-fluorophenyl)-3-(4H-1, 2, 4-triazol-4-ylamino) azetidin-2-one, 1c:- Yield 66%; M.P 1200C; IR: 1671 (C=O), 834 (C-Cl), 1621 (C=N), 1491 (C=C), 1520 (N-H), 3142 (C-H Stretching hetero aromatic); 1H NMR: 7.421-7.352 (m, Aromatic 8H), 7.3-7.152 (s, 2H, CH triazole), 8.381 (d, 1H, NH), 4.23(s, 1H, CH-Ar), 1.35(d, 1H, CH of azetidinone).
1-(4-chlorophenyl)-4-(4-methoxyphenyl)-3-(4H-1,2,4-triazol-4-ylamino)-azetidin-2-one, 2c:-Yield 67%; M.P 1330C, IR: 1632 (C=O), 842 (C-Cl), 1626 (C=N), 1452 (C=C), 1562 (N-H), 3173 (C-H Stretching hetero aromatic); 1H NMR: 7.542-7.382 (m, Aromatic 8H), 7.1-7.522 (s, 2H, CH triazole), 8.231 (d, 1H, NH), 4.17(s, 1H, CH-Ar), 1.31(d, 1H, CH of azetidinone).
1-(4-chlorophenyl)-4-(3-nitrophenyl)-3-(4H-1,2,4-triazol-4-ylamino)-azetidin-2-one,3c:- Yield 65%; M.P 1100C; IR: 1680 (C=O), 825 (C-Cl), 1613 (C=N), 1487 (C=C), 1549 (N-H), 3131 (C-H Stretching hetero aromatic); 1H NMR: 7.332-7.521 (m, Aromatic 8H), 7.2-7.032 (s, 2H, CH triazole), 8.271 (d, 1H, NH), 4.10(s, 1H, CH-Ar), 1.25(d, 1H, CH of azetidinone).MS: molecular ion peak is 383.8132and base peak is 260.0162.
4-(2-chlorophenyl)-1-(4-chlorophenyl)-3-(4H-1, 2, 4-triazol-4-ylamino) azetidin-2-one, 4c:- Yield 63%; M.P 1250C; IR: 1653 (C=O), 803 (C-Cl), 1643 (C=N), 1472 (C=C), 1593 (N-H), 3161 (C-H Stretching hetero aromatic); 1H NMR: 7.832-7.631 (m, Aromatic 8H), 7.1-7.042 (s, 2H, CH triazole), 8.941 (d, 1H, NH), 4.13(s, 1H, CH-Ar), 1.73(d, 1H, CH of azetidinone).
1, 4-bis (4-chlorophenyl)-3-(4H-1, 2, 4-triazol-4-ylamino) azetidin-2-one, 5c:- Yield 70%; M.P 1420C; IR: 1631 (C=O), 875 (C-Cl), 1698 (C=N), 1441 (C=C), 1502 (N-H), 3150 (C-H Stretching hetero aromatic); 1H NMR: 7.662-7.21(m, Aromatic 8H), 7.4-7.142 (s, 2H, CH triazole), 8.521 (d, 1H, NH), 4.09(s, 1H, CH-Ar), 1.23(d, 1H, CH of azetidinone).
Anti-tubercular activity
The synthesised analogues 1c, 2c and 3c were selected for antitubercular activity. Mycobacterium tuberculosis H37Rv maintained in Lowenstein Jensen medium was used as the test organism for anti-mycobacterial screening studies. The bacterial cultures were grown till mid-log phase in the Middle brook 7H9 broth for Mycobacterium tuberculosis H37Rv. Stock solutions of the test compounds were prepared at a concentration of 2 mg/ml. 50 μL of the mid-log phase culture was added to 150μL of the media taken in microtitre plates. From the stock solution of the compounds 1c, 2c and 3c was added to the wells to final concentration of 100, 250, 500 μg/ml. The control wells contained culture without any compound. All the tests were done in duplicates. The plates were then incubated at 37°C for 7 days. After incubation 20μL of Resazurin dye was added and change of colour, if any was noted. The control wells showed no change of colour from pink. Those compounds which prevented the change of colour of the dye from blue to pink were considered to be inhibitory [25]. It was found that all the selected azetidinone derivatives are active against Mycobacterium tuberculosis and from which analogue 2b and 2c showed profound activity against the organism at lower concentration. The MIC was defined as of the compounds were given in table 9.
This research was focused on the rational approach in design and development of azetidinone derivatives comprising 1, 2, 4-triazole for novel antitubercular agents. The present research work involved the preliminary in silico designing, synthesis, characterisation and biological screening. Azetidinone with 1, 2, 4-triazole moiety showed a better binding character than azetidinones. Out of the results obtained, it may be concluded that the3-nitro and 4-methoxy benzaldehyde analogues of azetidinone derivatives (2c and 3c) showed good receptor binding with the selected target sand good biological activity.
Authors are thankful to the Principal, Mr. P. Sri Ganesan, University College of Pharmacy, Mahatma Gandhi University, Kottayam, Kerala for providing research facilities.