e-ISSN:2320-1215 p-ISSN: 2322-0112
e-ISSN:2320-1215 p-ISSN: 2322-0112
N Rathna Prasanna*, C Triveni, R Soumya, BV Ramana, and G Nagarajan
r. KV Subba Reddy, Institute Of Pharmacy, Kurnool, Andhra Pradesh, India.
Received date: 07/02/2013 Accepted date: 25/03/2013 Revised date: 18/02/2013
Visit for more related articles at Research & Reviews in Pharmacy and Pharmaceutical Sciences
The development of drug delivery systems for cancer therapeutics has improved the therapeutic efficacy of existing chemotherapies and also resulted in the creation of new ones. A variety of these technologies are already in clinical use, including controlled delivery systems for cancer drugs, localized chemotherapy systems, polymer-drug conjugates, liposomal systems, and trans dermal drug delivery patches. The possibility of targeting the delivery of cancer drugs to tumor tissues may be realized by using specific polymer-drug conjugates or by manipulating the physicochemical properties of polymers or liposomal systems to match the tumor environment. A controlled release microchip has been developed that can dispense one or more drugs as needed and represents the next generation of ―smart‖ drug delivery approaches. Drug-delivery systems have had a growing influence on the clinical application of cancer therapeutics for several decades, and novel delivery systems now have been used by millions of patients.
Cancer, Chemotherapy, Novel, Delivery
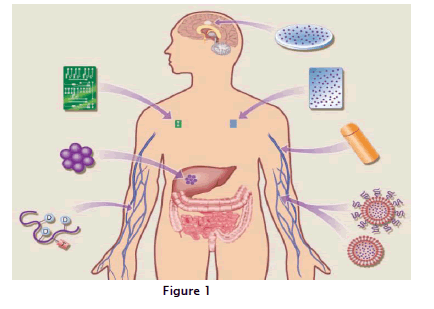
These delivery systems have enabled the development of new cancer therapies, have permitted cancer treatments with significantly reduced side effects and have facilitated the development of more effective chemotherapeutic regimens using anticancer drugs that are already available. In addition to their direct effects on treatments targeting tumor growth and progression, new drug delivery systems also have impacted the areas of cancer prevention and pain management associated with cancer chemotherapy. In this article, we review some of the drug-delivery systems that are FDA-approved and in clinical use against a variety of cancers. These systems include controlled delivery of cancer drugs, local chemotherapy, polymer-drug conjugates, liposomal systems and trans dermal drug-delivery patches. The possibility of delivering cancer drugs in a targeted manner with increased control has captured the imagination of scientists from a broad range of disciplines, including microelectronics, nanotechnology, material science, immunology and human genomics and their efforts have resulted in the development of the next generation of "smart" drug-delivery approaches. According to figure:1 A variety of drug-delivery approaches are being used clinically or are in development for the treatment of human cancers. Systems in clinical use include polymer microspheres carrying anticancer peptides, used to treat advanced prostate carcinoma (The drug is released through diffusion or polymer degradation); polymer wafers embedded with the chemotherapeutic agent BCNU, used for the localized treatment of brain cancer; osmotic pumps delivering antiangiogenic and other drugs; and liposomal systems, used in the treatment of Kaposi’s sarcoma. Polymer-drug targeting moiety conjugates are being tested against a variety of human cancers, and controlled-release microchips are in various stages of preclinical development.
Nanoparticles applied as drug delivery systems are submicron sized particles (3-200 nm), devices, or systems that can be made using a variety of materials including polymers (polymeric nanoparticles, micelles, or dendrimers), lipids (liposomes), viruses (viral nanoparticles), and even organmetallic compound (nanotubes; Table1).
a. Polymeric nano particles: polymeric nanoparticles in which drugs are conjugated to or encapsulated in polymers
b. Polymeric micelles: amphiphilic block copolymers that form to nanosized core/shell structure in aqueous solution The hydrophobic core region serves as are servoir for hydrophobic drugs, where as hydrophilic shell region stabilizes the hydrophobicore and renders the polymer to be water-soluble.
c. Dendrimers: synthetic polymeric macromolecule of nanometer dimensions, which is composed of multiple highly branched monomer, that emerge radially from the central core.
d. Liposomes: self-assembling structures composed of lipid bi layers in which anaqueous volume is entirely enclosed by a membranous lipid bilayer.
e. Viral-based nanoparticles: in general structure are the protein cages, which are multivalent, self-assembles structures.
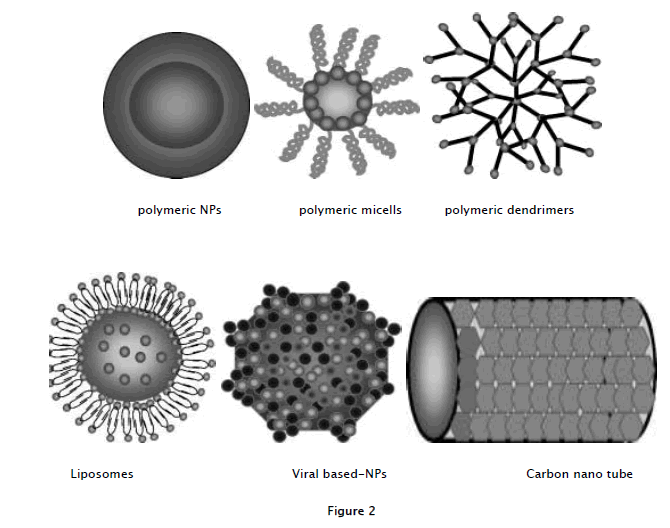
f. Carbon nanotubes: carbon cylinders composed of benzene rings. All these are represented in fig 2
CPX-1 is a novel liposome-encapsulated formulation of irinotecan and floxuridine designed to prolong in vitro optimized synergistic molar ratios of both drugs following infusion. An open-label, single-arm, dose-escalating phase I study was conducted to determine the maximum tolerated dose and pharmacokinetics of CPX-1 in patients with advanced solid tumors. The results showed that CPX-1 was well tolerated, and antitumor activity was shown in patients with advanced solid tumors.
MCC-465 is an immunoliposome-encapsulated doxorubicin (DOX). The liposome is tagged with polyethylene glycol (PEG) and the F(ab")2 fragment of human mAb GAH (goat anti-human), which positively reacts to >90% of cancerous stomach tissues but negatively to all normal tissues. In preclinical studies, MCC-465 showed superior cytotoxic activity against several human stomach cancer cells compared with DOX or DOX-incorporated PEG liposomes. A phase I clinical trial was conducted to define the maximum tolerated dose, dose-limiting toxicity, recommended phase II dose and pharmacokinetics of MCC-465. The results showed that MCC-465 was well tolerated, and the recommended dose for a phase II study was considered to be 32.5 mg/m2 in an equivalent amount of DOX. A phase II trial was recommended, but further information is currently not available.
Polymeric micelles are expected to increase the accumulation of drugs in tumor tissues utilizing the EPR (enhanced permeability and retention) effect and to incorporate various kinds of drugs into the inner core by chemical conjugation or physical entrapment with relatively high stability. The size of the micelles can be controlled within the diameter range of 20-100 nm to ensure that the micelles do not pass through normal vessel walls; therefore, a reduced incidence of the side effects of the drugs may be expected as a result of the decreased volume of distribution. There are several anticancer agent-incorporated micelle carrier systems under clinicalevaluation. Phase I studies of a CDDP (cisplatin) -incorporated micelle, NC-6004, and a phase II study of a PTX (Paclitaxel) -incorporated micelle, NK105 for stomach cancer, are in progress.
The antitumor activity of SP1049C, a novel P-glycoprotein targeting micellar formulation of doxorubicin consisting of DOX and two nonionic block copolymers, has been evaluated in patients with advanced adenocarcinomas of the esophagus and gastroesophageal junction. SP1049C had a notable single-agent activity in patients with adenocarcinomas of the esophagus and gastroesophageal junction, as well as an acceptable safety profile. These results, in addition to the results of preclinical studies, demonstrate superior antitumor activity of SP1049C compared with DOX in a standard formulation. Phase III clinical trials are now in progress.
The basic principle of targeted drug delivery in cancer using nanoparticles injected intravenously into the blood circulation is that the nanoparticles should recognize the receptors on the cancer cell, anchor themselves to it and diffuse inside the cell. Once inside, the nanoparticle disintegrates, causing a nearly instantaneous release of the drug precisely where it is needed. Nanoparticles can be chemically programmed to have an affinity for the cell wall of tumors or attached to mAb ligands that bind specifically to receptors on tumor cells. To be effective, the particles must evade the body's immune system, penetrate into the cancer cells and discharge the drugs before being recognized by the cancer cells.
Nanosystems are emerging that may be very useful for tumor-targeted drug delivery. Novel nanoparticles are preprogrammed to alter their structure and properties during the drug delivery process to make them most effective for delivery. This alteration can be achieved through the incorporation of molecular sensors that are able to respond to physical or biological stimuli, including changes in pH, redox potential or enzymes.
Nanoparticles are suitable for two specific tasks required for targeted drug delivery to pathological sites in the body: (1) passive enhanced permeability and retention based on the longevity of the pharmaceutical carrier in the blood, leading to its accumulation in pathological sites with compromised vasculature, and (2) active targeting based on the attachment of specific ligands to the surface of pharmaceutical carriers to recognize and bind pathological cells.. Differentially expressed molecules at receptors can be used as docking sites to concentrate drug conjugates and nanoparticles at tumor sites.
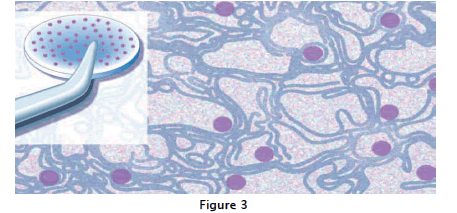
These problems were resolved by physically embedding the drugs into polymers, where they were released by a combination of diffusion through pores as well as polymer matrix degradation. This approach is the basis for the currently available injectable delivery systems (Lupron®, Zoladex®, Decapeptyl®) that now last from 1 to 4months and are used successfully by hundreds of thousands of patients with prostate cancer. Another effective method to extend drug lifetimes is by chemically binding the drug of interest towater-soluble polymers, such aspolyethylene glycol (PEG). This approach can decrease immunogenicity and extend the biological lifetime of the drug. Such systems have been used for the delivery of as par aginase, interferon, and granulocyte colony-stimulating growth factor (G-CSF).it is explained to table:2.
As shown in fig:3 it reveals the presence of pores left behind where released proteins had been.
In basic polymer systems, drug diffuses through the outside polymer rim, either through poresor a permeable membrane. Examples of this system include Norplant, Courser, and others. In the degradable matrix system, drug is evenly distributed and released by diffusion through the polymer or by a combination of diffusion and polymer erosion. In the osmotic system, the membrane is permeable to water but impermeable to drug; the movement of water through them embrane by osmosis, due to the presence of the drug itself or salts that have been encapsulated with the drug, then pumps the drug out through a tiny hole in the membrane. In polymer drug conjugates, a polymer (curved line) is connected to a drug via bonds that can be cleaved once inside the body. Some of these conjugates contain a targeting (T ) mmoiety as well, explained in fig:4.
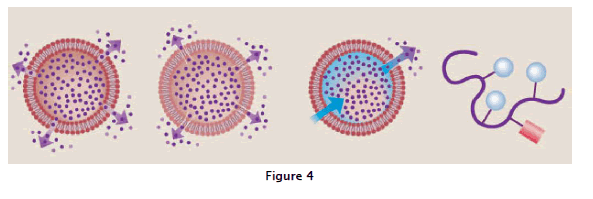
Most commonly used cancer chemotherapy agents induce significant toxicity that ranges in severity from uncomfortable to life threatening. To avoid this problem, approaches have been developed to deliver these drugs locally, with the goal of improving both their safety and efficacy. Local delivery guarantees higher local drug concentrations when compared to those obtained with traditional systemic delivery methods. Delivery of a polymer-drug conjugate during cancer surgery would have the advantage of enhancing the benefit of surgery while minimizing the systemic toxicity that is usually associated with standard drug treatments. This approach has now been successfully exploited, most notably in brain cancer surgery. The ability to deliver chemotherapeutic drugs to this organ via traditional systemic routes is severely restricted by the presence of the blood-brain barrier, which limits transport to this organ. Novel polymers, such as polyanhydrides in the form of wafers, have now been used to deliver chemotherapeutic drugs, such as carmustine (BCNU), locally to treat brain cancer.
In 1987, Henry Brem and colleagues inserted these drug-impregnated wafers, layered at the surface of the brain, into the tumor section cavity following tumor removal. The drug is slowly released from these wafers for approximately 3 weeks to destroy any residual tumor. Because the drug is delivered locally, rather than systemically, harmful side effects that normally occur with carmustine were minimized. Results from one of a number of clinical trials showed that 31%of the patients treated in this manner were alive after 2 years, in comparison to only 6% of patients receiving standard brain tumor therapies. In 1996, this therapy was approved by the U.S. FDA for patients with recurrent glioblastoma, the first new brain cancer therapy.
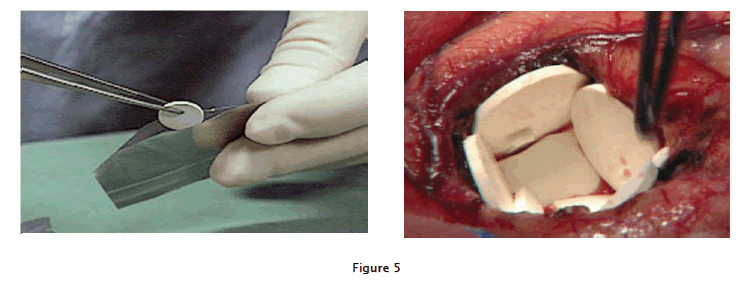
Polymer implants containing carmustine are inserted into a human brain, lining the tumor resection cavity, where the loaded drug is gradually released as the polymer wafers dissolve approved in over 20 years. In 2003,the FDA extended its approval to include initial surgery for malignant glioma, based on two additional and omized prospective studies that demonstrated improved survival and safety. Experimental brain metastases and invasive pituitary adenomas also have been treated successfully by this approach. Carcinomatousmeningitis likewise has been treated using long-lasting, implantable lipid formulations to deliver cancer drugs to spinal fluid, well explained in fig:5.
Cancer drug targeting is a rapidly developing research discipline that has recently yielded a number of different drug-delivery approaches. One such strategy has been to couple small-molecular-weight cancer drugs to polymers. This coupling results in an altered bio distribution of the drugs following intravenous administration that favors concentration of the drug in tumors. Normally, low-molecular-weight anticancer drugs will nonselective lypenet rate most tissues, because they pass rapidly through cell membranes. This results in a relatively rapid distribution of the drug with no tumor selectivity. In the case of polymer-drug conjugates, however, the polymer-drug linkages are designed to be stable in the bloodstream. This feature, along with the fact that the higher molecular-weight polymer-drug can only gain entry into cells via endocytosis, results in circulation of the polymer-drug for a longer period than with the drug alone. Because most normal tissues have non leaky micro vasculatures, the polymer-drug accumulates more in tumor tissue, which has a notoriously leaky vascular supply. In one example of this approach, Ruth Duncan and her colleagues developed a conjugate of the polymer N-(2-hydroxypropyl) methacrylamide(HPMA copolymer) to doxorubicin that could be cleaved by specific enzymes in lysosomes, resulting in the selective release of the drug. This approach resulted in the concentration of approximately70 times more doxorubicin in mouse melanoma tumors than in normal tissues. Importantly, this approach also increased the maximum tolerated dose of the polymer-drug by up to 10 times that of the free drug. Tumor-specific polymer-drug conjugates can also be created by adding specific targeting moieties to the polymer to aid in treatment of specific tumors (e.g., adding galactosamine to target hepatocellular carcinoma). There are currently10 different polymer-drug systems at various stages of clinical trials. The use of small lipid vesicles called liposomes also has been investigated as a means of using drug delivery to alter drug distribution. In this case, drugs are encapsulated inside liposomes, which circulate freely in the blood stream. The drug is emitted via diffusion through the liposome or by liposomal degradation. Encapsulation of the drug in a liposome reduces many of the side effects associated with certain anti cancer agents, such as cardiac toxicity, by preventing release of the drug at undesirably high concentrations as would occur with simple injection. A significantly high drug-carrying capacity also can be achieved using liposomes as opposed to attaching a drug to a single polymer chain. One area of active research involves the attachment of cancer cell-targeting ligands to liposomes in order to direct the liposomal drugs preferentially to cancer cells. Several key issues must be resolved before the full promise of liposome-based delivery can be realized. One of these is the prevention of liposomes from accumulating and being cleared by phagocytic cells. Among the approaches being investigated to prevent this involves attaching polyethyleneglycol to the liposomes (PEGylated liposomes).Liposomal drug-carrier systems with daunorubicin and doxorubicin have now been approve for the treatment of associated Kaposi’s sarcoma, and liposomal amphotericin B has been approve for the treatment of HIV-associated Kaposi’s sarcoma, and liposomal amphotericin B has been approved for the treatment of fungal infections in cancer.
Small lipophilic drugs have shown to have the ability to cross the skin quite efficiently. A variety of transdermal patches have now been developed, tested, and approved for several different drugs and conditions. These patches are composed of polymers impregnated with drug that diffuse through the polymer and skin to reach the systemic circulation.
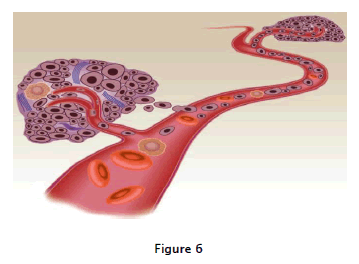
As shown in fig:6 Growth and metastasis in a solid tumor depend on angiogenesis, the formation of new capillaries from an existing vessel. The tumor will not grow to more than a few millimeters in diameter without being vascularized and oncevasculaization occurs, tumor growth is often exponential. Metastasis may occur through direct extension or hematogenousspread, but angiogenesis also is required for metastases to become established as growing tumors. the transdermal patches currently available are scopolamine for motion sickness, nitroglycerine for angina, fentanyl for pain, and clonidine for hypertension.
Transdermal delivery also has played an important role in both cancer therapy and prevention. Most compelling is the use of nicotine patches in preventing smoking and prolonging life. As described by Henning field in1995, 2 years after they were on transdermal nicotine patches for 12 weeks, four times as many patch wearers did not smoke as compared to those who received placebos. Based on these studies, it can be estimated that over one million smokers to date in the U.S. have given up smoking due to the use of these patches. In addition, transdermal delivery systems for fentanyl are used to relieve pain in cancer patients. These systems last for 3 days and are used by hundreds of thousands of patients each year.
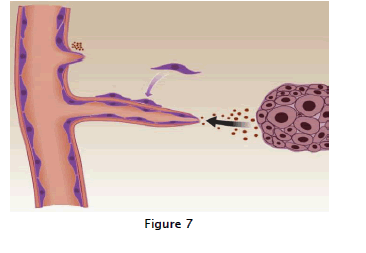
In the last several decades, the development and use of controlled release polymers has enabled the design of bioassays for the in vivo identification and testing of angiogenesis inhibitors. This, in turn, has led to the introduction of a significant group of new cancer therapeutics, which target the new capillary growth that invades and nurtures developing tumors. This antiangiogenic strategy to treat human cancer, pioneered by Judah Folk man, was recently validated in a large randomized clinical trial. In this trial, reported by Hurwitz and coworkers, bevacizumab(Avastin), a vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) antagonist, was administered along with conventional chemotherapy and significantly improved the survival of patients with metastatic colorectal Cancer. Bevacizumab, a recombine anthumanized anti-VEGF monoclonal antibody, was given in combination with the standard chemotherapy of irinotecan, fluorouracil, and leucovorin (IFL) to a cohort of over800 patients. Patients who received the combination therapy showed a median survival of 20.3 most compared to 15.6 most for the placebo group. Studies aimed at achieving maximum therapeutic efficacy of some antiangiogenic drugs have led, ironically, to the development of what is now referred to as per fig:7 a series of cellular and biochemical events is required for tumor-induced angiogenesis. The solid tumor releases angiogenic mitogens, such as vascular endothelial cell growth factor (VEGF)and fibroblast growth factor (FGF),which stimulate the sprouting of a new capillary vessel and initiate the chain of events required to create an intact capillary.
"Antiangiogenic scheduling" of conventional chemotherapies. This regimen is able to circumvent the drug resistance induced by these same anticancer drugs delivered on the traditional chemotherapeutic schedule. The first example of this phenomenon was seen in studies with the chemotherapeutic agent cyclophosphamide, when it was delivered on a low-dose, high-frequency schedule as opposed to standard bolus administration. This schedule resulted in effective control of tumor growth, with a concomitant lack of drug resistance in a number of tumor models. This delivery schedule, termed "low-dose metronomic chemotherapy" is currently being tested in clinical trials. The unique vasculature of tumors, characterized by increased permeability and their complex 3-D architecture, has recently been exploited as an approach to deliver tumor-suppressing drugs with increased efficiency. By manipulating the physicochemical properties of physiology features of a tumor (e.g., low pH), so that the liposomes release their carried drug selectively in tumor tissue. Liposome-mediated delivery of anticancer drugs has improved significantly with a concomitant increased accumulation of drug in tumor vessels.
The tumor endothelium has proven to be an exciting target for anticancer drugs whose goal is to stop the angiogenesis required for tumor growth and progression. Many of the first-generation antiangiogenic proteins in clinical testing are delivered systemically and, for the most part, target active endothelium, such as that feeding a solid tumor, as opposed to the quiescent endothelium that supports normal, healthy tissues. If these angiogenesis inhibitors could be selectively targeted to the metabolically active endothelium of tumors, a much higher therapeutic index could be achieved.
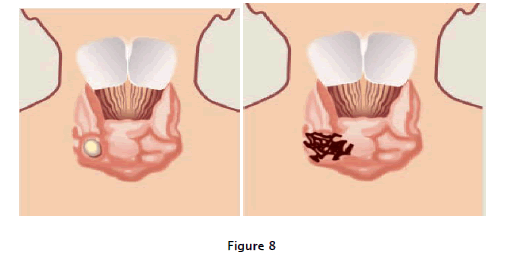
Fig: 8 explain that Angiogenic switch" refers to the stage at which a small, a vascular, benign tumor acquires its own vasculature. This small thyroid tumor is shown before and after it has acquired the angiogenic phenotype. Tumors that have "switched on" angiogenesis are capable of rapid expansion and metastasis.
This goal recently was achieved with the development of a water soluble polymer system used to deliver an antiangiogenic agent,TNP-470, to the tumor microvasculature.TNP-470, a low-molecular weight analogue of fumagillin, was first shown to be antiangiogenic in 1990 by Ingber and colleagues. More recently, when tested in clinical trials against a variety of tumors, TNP-470 treatment showed promising antitumor activity when used alone or in combination with conventional chemotherapy. However, the promise of this drug was significantly limited by neurotoxicity that occurred at the optimal anticancer dose. Using an approach that combines a drug polymer complex with targeted delivery to the neovasculature, Satchi-Fainaro and Folk man were able to achieve enhanced and prolonged activity of TNP-470 in a variety of in vivo models. They designed and synthesized a water-soluble conjugate of N-(2-hydroxypropyl)methacrylamide(HPMA) copolymer, a Gly-Phe-Leu-Gly linker, and TNP-470.This conjugate selectively accumulated in the tumor microvasculature due to the passive targeting phenomenon first described by Matsumura and Maeda. Called the enhanced permeability and retention(EPR) effect, this accumulationis attributed to the increased permeability and absence of effectively mphatic drainage in tumors.
The HPMA copolymer-TNP-470conjugate, in addition to potently inhibiting tumor angiogenesis and subsequent tumor growth, did not cross the blood-brain barrier and did not induce neurotoxicity as did the un conjugated drug. Approaches such as these hold significant promise for the development of new targeted antiangiogenic therapies as well as for the optimization of existing antiangiogenic drugs. Current interest is focusing on an even earlier stage in tumor progression, the point at which a dormant, avascular tumor acquires the ability to grow and metastasize by "switching on" angiogenesis. As sensitive biomarkers and imaging systems capable of detecting the nascent microvasculature are developed, it is possible to imagine using a potent angiogenesis inhibitor that targets the first generation of angiogenic vessels developing in a tiny tumor lesion that is in the process of acquiring the angiogenic phenotype. Such an agent might be capable of maintaining the dormancy of that lesion indefinitely. Much remains to be accomplished in our ability to target and deliver chemotherapeutic agents to a growing tumor. For example, successful localized delivery of drugs is still limited by less than optimal drug diffusion within cancerous tissues, as well as by occasional undesirable interactions between drug and delivery vehicle. Smarter drug delivery systems are being developed to address some of these challenges. A controlled-release microchip that stores and delivers on demand many different drugs (e.g., "pharmacyon a chip") has recently been developed by John Santini and Robert Langer. Such a system can eventually be preprogrammed or externally regulated to release drugs at anytime, pattern, and rate. This ability would, in turn, facilitate the delivery of novel combination therapies, permitting the targeting of endothelial cells via the use of angiogenesis inhibitors, followed by suppression of the remaining tumor cells with chemotherapeutic drugs and subsequent maintenance on antiangiogenic therapy. Ultimately, equal attention to the development of more potent and specific cancer chemotherapeutics, as well as to the optimal systems to deliver these drugs, will be necessary to guarantee that patients with cancer benefit from these advances.
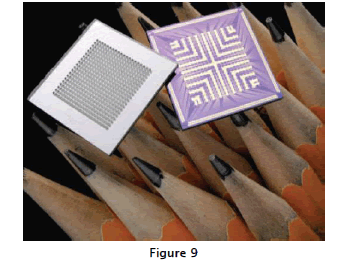
Implantable drug-releasing microchips can deliver hundreds of doses over their lifetimes, lasting from months to years (Microchips, Inc., Bedford, MA).In some models, the various reservoirs can store one or multiple drugs, to be released on demand via a preprogrammed schedule or external control,represented in fig;9
Enhanced permeability and retention effect. Nano particles that satisfy the size and surface characteristics requirements described above for escaping reticulo endothelial system capture have the ability to circulate for longer times in the blood stream and a greater chance of reaching the targeted tumor tissues. The unique pathophysiologic characteristics of tumor vessels enable macromolecules, including nano particles, to selectively accumulate in tumor tissues . Fast-growing cancer cellsvessels near the tumor mass to supply them with oxygen and nutrients . The resulting imbalance of angiogenic regulators such as growth factors and matrix metallo proteinases makes tumor vessels highly disorganized and dilated with numerous pores showing enlarged gap junctions between endothelial cells and compromised lymphatic drainage. These features are called the enhanced permeability and retention effect, which constitutes an important mechanism by which macromolecules, including nano particles, with amolecular weight above 50 kDa, can selectively accumulate in the tumor interstitium (Fig. 2;).Tumor microenvironment. Another contributor to passive targeting is the unique microenvironment surrounding tumorcells, which is different from that of normal cells. Fast-growing, hyper proliferative cancer cells show a high metabolic rate, and the supply of oxygen and nutrients is usually not sufficient for them to maintain this. Therefore, tumor cells use glycolys is to obtain extra energy, resulting in an acidic environment. The pH-sensitive liposomes are designed to be stable at a physiologic pH of 7.4, but degraded to release active drug in target tissues in which the pH is less than physiologic values ,such as in the acidic environment of tumor cells . Additionally, cancer cells express and release unique enzymes such as matrix metalloproteinases, which are implicated in their movement and survival mechanisms. An albumin-bound form of doxorubicin incorporating a matrix metalloproteinase-2–specific octapeptide sequence between the drug and the carrier was observed to be efficiently and specifically cleaved by matrix metalloproteinase-2 in an in vitro study.
A drug delivery system comprising a binary conjugate (i.e., polymer-drug conjugate) that depends only on passive targeting mechanisms inevitably faces intrinsic limitations to its specificity. One approach suggested to overcome these limitations is the inclusion of a targeting ligand or antibody in polymer-drug conjugates. Initially, direct conjugation of ananti body to a drug was attempted. However, in clinical trials conducted thus far, such early antibody-drug conjugates have failed to show superiority as a targeted delivery tool for the treatment of cancer. One of the reasons for this is that the number of drug molecules that can be loaded on the antibody while preserving its immune recognition is limited.
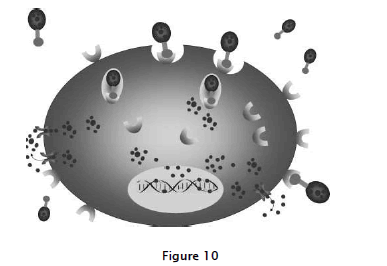
Internalization of nanoparticles via receptor-mediated endocytosis. Tumor-specific ligands or antibodies on the nanoparticles bind to cell-surface receptors, which trigger internalization of the nanoparticles into the cell through endosome. As a pH value in the interior of the endosome becomes acidic, the drug is released from the nanoparticles and goes into the cytoplasm. Drug-loaded nanoparticles bypass the P-glycoprotein efflux pump not being recognized when the drug enters cells, leading to high intracellular concentration, well explained in fig:10
Tumor-specific ligands or antibodies on the nano particles bind to cell-surface receptors, which trigger internalization of the nano particles into the cell through endosome. As a pH value in the interior of the end some becomes acidic, the drug is released from the nano particles and goes into the cytoplasm. Drug-loaded nano particles bypass the P-glycoprotein efflux pump not being recognized when the drug enters cells, leading to high intracellular concentration.The recent development and introduction of a wide variety of liposomes and polymers as drug delivery carriers increases the potential number of drugs that can be conjugated to targeted nano particles without compromising their targeting affinity relative to earlier antibody-drug conjugates. Taking advantage of this array of carriers, targeting moieties, and drugs, many recently eveloped active targeting drug conjugates use a ternary structure composed of a ligand or antibody as a targeting moiety, a polymer or lipid as a carrier, and an active chemotherapeutic drug. When constructing ternary structure nano particles, some factors must be considered to create more efficient delivery systems. Antigen or receptor expression. Ideally, cell-surface antigens and receptors should have several properties that render them particularly suitable tumor-specific targets . First, they should be expressed exclusively on tumor cells and not expressed on normal cells. Second, they should be expressed homogeneously on all targeted tumor cells. Last, cell-surface antigens and receptors should not be shed into the blood circulation. Internalization of targeted conjugates. Whether targeted conjugates can be internalized after binding to target cells is an important criterion in the selection of proper targeting ligands. Internalization usually occurs via receptor-mediated endocytosis (Fig. 3). Using the example of the folate receptor, when afolate-targeted conjugate binds with folate receptor on the cell surface, the invaginating plasma membrane envelopes the complex of the receptor and ligand to form an endosome.Newly formed endosomes are transferred to target organelles. As the pH value in the interior of the endosome becomes acidiclysozymes are activated, the drug is released from the conjugate and enters the cytoplasm, provided the drug has the proper physico-chemical properties to cross the endosomalmembrane. Released drugs are then trafficked by their target organelle depending on the drug. Meanwhile, the folatereceptor released from the conjugate returns to the cell membrane to start a second round of transport by binding with new folate-targeted conjugates.
The folate receptor is a well-known tumor marker that binds vitamin folate and folate-drug conjugates with a high affinity and carries these bound molecules into the cells via recept ormediated endocytosis. We have checked the incidence offolate receptor expression in human head and neck primary and metastatic tumor tissues and compared them with normal tissues such as the bone marrow. Folate receptor expression was found in 53% of these tumor samples whereas normal bone marrow cells did not show any folate receptor expression .Recently, we generated a new folate receptor–targeted nano particle formulation of paclitaxel using heparin as a carrier[heparin-folate-Taxol (paclitaxel), HFT] and tested it using nudemouse animal models. This novel ternary nano particle HFT showed more potent activity against the growth of tumor xenografts of human KB and paclitaxel-resistant KB derivatives than did binary heparin-Taxol or free drug (paclitaxel; )Aptamers are oligonucleic acids such as DNA or RNA that bear unique threedimensional conformations capable of binding to target antigens with high affinity and specificity. They have been applied to drug delivery systems as aligand to enhance selectivity.
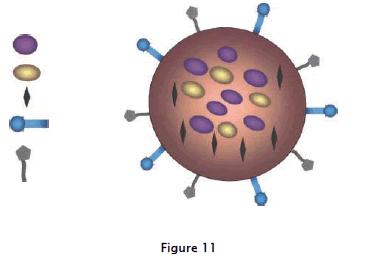
The following are illustrated based on fig:11 the ability to carry one or more therapeutic agents; bio molecular targeting through one or more conjugated antibodies or other recognition agents; imaging signal amplification, by way of co-encapsulated contrast agents. These nano particles will eventually be capable of detecting malignant cells (active targeting moiety), visualizing their location in the body (real-time in vivo imaging), killing the cancer cells without side effects by saving normal cells (active targeting and controlled drug released system or photo thermal ablation), and monitoring the treatment effect in real time.
The in vivo efficacy of Docetaxel-encapsulated poly(lactic-co-glycolic acid) nano particle conjugated with an aptamer to target prostate-specific membrane antigens was evaluated using an animal model .Transfer in, a serum glycoprotein, works as a transporter to deliver iron through the blood and into cells by binding to thetransferrin receptor and subsequently being internalized via receptor-mediated endocytosis . Because the transfer in receptor is over expressed in tumor tissues compared with normal tissues, it has been investigated as a target for tumor specific drug delivery . Transfer in-conjugated paclitaxel loaded [poly(lactic-co-glycolic acid) polymer] nano particles displayed greater inhibitory effects on cell growth than freepaclitaxel in MCF-7 and MCF-7/Adr cells . Transferrin wasalso conjugated to liposomes to increase the transaction efficacy of p53, resulting in the sensitization of the transfected cancer cells/xenografts to ionizing radiation.Lectins are proteins that recognize and bind to carbo hydratemoieties attached to protein molecules (glycans) on the extracellular side of the plasma membrane. Cancer cells often express different glycans compared with their normal counterparts. Therefore, lectins could be used as targeting molecules to direct drugs specifically to desired cells and tissues . This protein (lectin)-carbohydrate interaction can be applied to develop two types of nanoparticles: one incorporates lectins into nano particles as targeting moieties that are directed to cell surface carbohydrates (direct lectin targeting), and the other is are verse scenario in which carbohydrate moieties are coupled to nano particles to target lectins (reverse lectin targeting; .An example of a drug conjugate using this particular reverse selection targeting is PK2, an actively targeted variant of the already developed PK1, in which the targeting moiety galactosamine is attached to the polymer backbone. Gamma-camera imaging showed that the PK2 targeting conjugate effectively targeted the liver whereas the non conjugated counterpart (PK1) showed not targeting. Phase I/II clinical trials have been completed in patients with primary or metastatic liver cancers.
The rationale for using nanobiotechnology in oncology is that nanoparticles have optical, magnetic or structural properties that are not available from larger molecules or bulk solids. When linked with tumor-targeting ligands such as mAbs, peptides or small molecules, these nanoparticles can be used to target tumor antigens (biomarkers) as well as tumor vasculatures with high affinity and specificity . Nanoparticles measuring 5-100 nm in diameter have sufficiently large surface areas and functional groups for conjugating to multiple diagnostic and therapeutic anticancer agents. Recent advances have led to bioaffinity nanoparticle probes for molecular and cellular imaging, targeted nanoparticle drugs for cancer therapy and integrated nanodevices for early cancer detection and screening. Nanobiotechnology has contributed significantly to the diagnosis and therapy of cancer and, by enabling a combination of these, will facilitate the development of personalized management of cancer. Nanobiotechnology has facilitated the discovery of biomarkers that can be used to diagnose and treat cancer based on the molecular profiles of individual patients. Nanoparticles enable targeted delivery of cancer therapeutics and increase efficacy as well as reduce adverse effects. Many challenges still remain to be resolved prior to widespread use of nanobiotechnology in clinical oncology. There is some concern about the toxicity of nanoparticles, and extensive investigations are in progress to resolve this issue. There is no consensus on the real risks of nanomaterials. Risk evaluation presents challenges because of a lack of data, the complexity of nanomaterials, measurement difficulties and undeveloped hazard assessment frameworks. This topic is discussed in detail in a chapter of a special report on nanobiotechnology .Detailed discussion is beyond the scope of this article, but some of the conclusions of this review are listed below: 1). The risk of nanoparticles depends on their type; some are toxic, whereas others have negligible toxicity and some even have a tissue-protective effect. 2) Measures are available to reduce the toxicity of nanoparticles.3). The use of biodegradable polymer nanoparticles is suitable for drug delivery as there is no significant toxicity.
Nanooncology has a promising future, and further advances are anticipated in the next 5 years. It is feasible to use molecular tools to design a miniature robotic device, a nanobot, that can be introduced in the body to locate and identify cancer cells and finally destroy them. The device would have a biosensor to identify cancer cells and a supply of anticancer substance that could be released on encountering cancer cells. A small computer could be incorporated to program and integrate the combination of diagnosis and therapy and provide the possibility of monitoring the in vivo activities by an external device. Since there is no universal anticancer agent, the computer program could match the type of cancer to the most appropriate agent. Such a device could be implanted as a prophylactic measure in people who do not show any obvious manifestations of cancer. It would circulate freely and could detect and treat cancer at the earliest stage. Such a device could be reprogrammed through removal control and enable a change of strategy if the lesion encountered is other than cancer.