In modern research growth of newer material copper sulphide is a need today for electronically and electrically active devices. A structural optical and Band gap determination signifies its characterisation, the band gap of these crystal exhibits variety of applications in semiconductor and in electronics .A crystal of copper sulphide shows purely semiconducting properties which are grown using hydrogen sulphide gas. A spectrum sensing advances Applications using X-ray diffraction.
Keywords |
| Copper, Band gap, Spectrum, Characterisation. |
INTRODUCTION |
| A characterization of growing crystal was performed using structural analysis and thermal Analysis. The Non linear
optical properties of various copper crystals is very important .In the recent year this prominent NLO behavior is
studied by growing iodated of alkali transition and alkaline earth material. it must be alkaline earth material. The band
gap of copper sulphide crystal exhibits variety of applications in semiconductor and in electronics .A crystal of copper
sulphide shows purely semiconducting properties which are grown using hydrogen sulphide gas. A spectrum of these
are discussed in this paper. It is also reported that sulphide are semiconductor in nature the synthesis of sulphide are
complicated because of relative low vapour pressure of the sulphur ,neverth less successively more attention is being
turns toward investigating sulphide of various materials .There is no need to explain the role of semiconductor in the
development of present electronic industries and semiconductor device for e.g. Zink sulphide (ZnS) used for lenses
and other optical devices lead sulphide used in infra, red sensors ; cadmium sulphide [cds] which is used in photocell.
Iodated, iodides and sulphide are taken it basic material copper. The growth of newer Engineering Semiconducting
material in the form of crystal is widely useful in different technologies. Some grown crystals having optical,
piezoelectric and NLO behavior. Copper sulphide crystals are insoluble in water which is decomposing before melting
point. In the work reported have the determination of the optical band gap of the sulphide crystal, its structural and
thermal behaviour explained and calculated. Copper sulphide is a metallic conductor due to the incomplete occupancy
of the sulphur, the determination of properties of copper sulphides is generally monovalent copper compounds.
Research on thin film solar cells with copper sulphide doped in iron, indium, cadmium, bismuth is a great interest
today. |
LITERATURE SURVEY |
| Today Crystal have became the base of modern technology in all aspects & ever increasing the demand of crystals in
the variety of field in science & technology. The work of growth & the study of copper iodated crystal have been taken
in the laboratory. The advances in the material science & solid state devices depend on the availability of good quality
crystals. Gel technique is considered more useful at room temp. & has an inexpensive Process |
EXPERIMENTAL PROCEDURE |
| A copper sulphide crystal are grown using chemical reaction method. Using this method gel of copper chloride formed
and water containing H2S gas filled with subsequent nucleation and the crystal growth of copper sulphide takes place. |
PREPARATION OF GEL |
| Initially different concentration solution of sodium Meta silicate taken for e.g. 10gm,12gm ,14 gm, 16 gm, 18 gm,
21gm, 22gm in distilled water to get 250cc solution. The solution is constantly stirred and then filtered by Dr Watts
filter paper. It is then kept in to an airtight bottle free from dust and contamination. Density of the solution was
measured using specific gravity bottle. A solution of different molarities prepared by adding proper amount of
chemicals to the double distilled water for copper nitrate, copper chloride, Hydrogen sulphide gas, acetic acid and
sodium meta silicate. A gel formation of mineral or organic acid takes place with a mixing of sodium meta silicate
solution. It forms process of polymerization in the mixture of solution or resultant solution. In the present work, various
concentration of acetic acid and those of sodium meta silicate were tried for optimum condition with different
concentrations of Hydrogen Sulphide gas solution |
SINGLE DIFFUSION TECHNIQUE |
| In actual procedure, 5cc of 2N acetic acid was taken in a small beaker, to which sodium Meta silicate solution of
density 1.04 gm/cc was added drop wise from burette with constant stirring performed with the help of magnetic stirrer,
till pH of the mixture reaches a value 4.4. A pH meter HANNA instrument of digital pocket sized is used for this
purpose. A 5cc of copper chloride solution of concentration 0.4M was added with constant stirring in mixture of acetic
acid and sodium meta silicate solution .A continuous stirring process required to avoids excessive ion concentration
which otherwise causes premature local gelling and makes the final medium inhomogeneous and turbid .The pH of the
mixture was maintained at 4.4 Number of attempts were tried for optimum condition for appropriate range of pH values
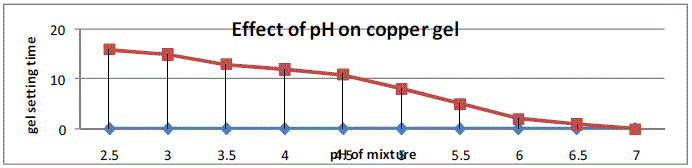
and allowed gel to obtain crystals of copper sulphide. The gel setting time required for the silica gel solution of pH
greater than 4.5was short, it is observed that the mixture of solution with pH value less than 4.2 required quite greater
number of days, however in the pH range 4.2 to 4.4 there is appropriate waiting in gelation time. Room temperature
and atmospheric effect also plays an important role on gelation, aging that is evaporation of water molecules form on
surface of gel. To perform these experiment borosil glass test tubes of diameter 2.5cm and height 25cm was used as
crystallizing vessels. This mixture was then transferred to the test tube, a mouth of test tube closed using cotton plug.
which is used to avoid contamination and dust affecting from atmosphere. The gel setting time was 12 to13days .This
completely set gel was left for aging for 4days.i.e.96 hour’s to120 hours .It is also observed that the aging of gel
reduces the diameter of the capillaries in gel so that reaction can be controlled. H2S gas dissolved in distilled water was
used as supernatant. |
EFFECT OF VARIOUS PARAMETERS ON CRYSTAL GROWTH OF COPPER SULPHIDE |
| It is necessary to study the effect of various parameters on crystal growth which is mainly depends on gel cell size, and
cell size is influenced by gel density etc. Hence, these parameters have profound influence on nucleation density,
growth rate habit and quality of crystals. Concentration of reactants is also important. |
EFFECT OF GEL DENSITY |
| The gels of different densities were obtained by mixing sodium Meta silicate solutions of specific gravity 1.02 to 1.08
with 2N acetic acid, keeping pH value constant. It was observed that transparency of the gel decreased with increase of
gel density gels with higher densities required less setting time of gel compared to the gels with lower densities. It may
be noted that well defined and transparent crystals were obtained with sodium Meta silicate solution of density
1.04gm/cc. On the other hand, gels with densities below 1.04 gm/cc required longer time to set and still gels were not
stable .Density of 1.02 gm/cc was the lower practical limit .The effect of gel densities on the quality of crystals as
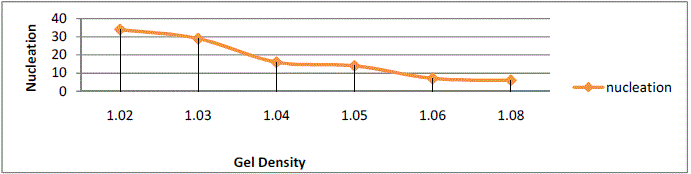
shown in table 5.5. The variation of gelation time with gel density shown in figure5.8 .It is observed that the gelation
time decreases with increase in gel density .Table 1 shows the effect of density on number of nuclei formed. A greater
gel density implies smaller pore size and poor communication among the pores and thus decreasing the nucleation
density. Bechhold et al showed that diffusion coefficient becomes distinctly smaller as gel densities increased. There is
no evidence that the diffusion constant of small atoms was greatly influenced by the silica gel density as long as the
density is low. Thus, the diffusion constant is not greatly influenced by the presence of dilute gel. |
EFFECT OF GEL AGING |
| Gel aging plays an effective role on the growth of copper sulphide crystals .To investigate the effect of aging
on gels, gel of same pH and density were allowed to age for various periods before adding the feed solution over a set
gel containing copper chloride. Supernatant of constant molarities was then added as a feed solution over the set gel. It
was found that number of copper sulphide crystal decreases with increase in aging of gel. Aging of gel decreases the
pore size as well as diffusion and nucleation density. More aging causes more amount of water evaporation out of the
gel. The effect of water evaporation should be considered before and after the formation of gel framework. Before the
gel is set the evaporation of water causes an increase in gel density which in turn decreases the diffusivity of reactive
sulphide ions in the gel, thereby decreasing the number of nucleation sites. Table-2.shows the effect of aging time on
number and the quality of crystal . In present work, aging of 120 hours was found suitable because it makes gel neither
dry or brittle nor fragile. The aim of reduction in nucleation centres can also be achieved. Hence aging period of 120
hours is the optimum condition for the growth of good quality crystals. |
X-RAY DIFFRACTION (XRD) |
| X-ray diffractogram is useful in the analysis of crystal structure, d-values, cell parameters, unit cell volume and lattice
system etc. can be evaluated using X-ray diffractogram. When the high frequency electromagnetic waves are selected
to have wavelength comparable to the interplaner spacing of the crystals, they are diffracted according to the physical
laws. The inter planer spacing (d) can be calculated to four digits and even more significant figures by measuring the
diffraction angles. This, in turn, can be used to determine cell parameters and the system to which the sample under
study belongs, etc. the reflecting planes in crystal h, k, l values can be calculated. |
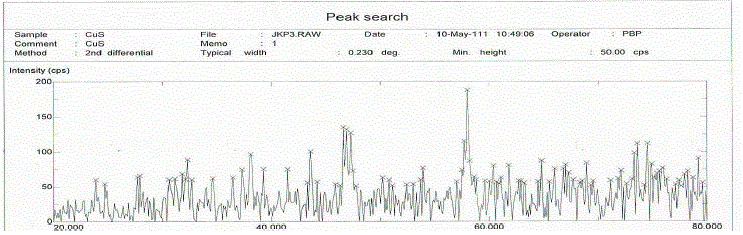
| X-ray diffractogram of gel grown crystal of copper sulphide was recorded using Minislex model ,Japan with Cukα
radiation of wave length 1.5408°.A and scanning speed of 10 0/minute. A copper target and nickel filter were used
From the powder diffractogram on data of copper sulphide which shows twenty different peaks and corresponding d
values and (h k l) values were computed by using computer program POWD [An interactive powder diffraction data
interpretation and indexing program] The recorded X-ray diffractogram is as shown in fig. 4. The present work
describes the characterization of copper sulphide crystals by Following Techniques. the X-ray and Thermal behaviour
of samples were practically performed at Jalgaon in North Maharashtra University Maharashtra. |
| These values are computed using computer programmed, POWD is as shown in the table 5.8. From POWD it found the
lattice parameter of unit cell satisfy condition a#b#c and α=β=90 and γ#90.so unit cell structure is monoclinic.
Calculated unit cell lattice parameter of the copper sulphide crystal. |
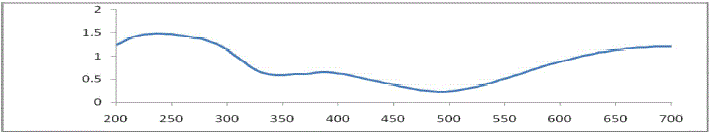
BAND GAP DETERMINATION - UV-VIS SPECTROPHOTOMETRY |
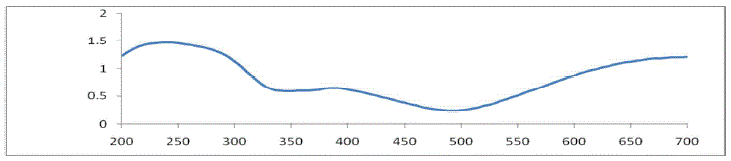
| The optical property of copper sulphide crystal can be studied using UV-VIS spectrophotometer. A fine powdered form
of copper sulphide crystal was used as sample .The reflection and absorption spectra of copper sulphide crystals have
been recorded over the wavelength range 200 to 700mm using a UV-2450 spectrophotometer of SHIMADZU scientific
instruments at the room temperature. The experiment was carried out in the research laboratory of the physics
department at Pratap College, Amalner Maharashtra. |
CONCLUSION |
| The Crystal of Semiconductor Copper Sulphide Can Be Grown by Using Gel Technique .Single Diffusion Gel Growth
Technique Is Suitable Copper Sulphide Crystals.Different Habits Of Copper Sulphide Crystals Can Be Obtained By
Changing Different Parameters In Crystal Growth.Unit Cell Parameter Value And D Values Match Very With The
Reported OnesThe Structure Of Copper Sulphide Is Monoclinic Confirmed By X-Ray Diffraction. |
| Fundamental Infra-Red Frequencies, Generally Observed In All Sulphide Compounds.A Band Gap Determination
Shows Variety Of Applications In Electronics And Electrical Devices And For Engineering Materials. |
Tables at a glance |
|
|
Figures at a glance |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|
References |
- Alipio BC, David FC, Isabel RS, Juan CNM. Manuel AE. Comparison of batch, stirred flow chamber, and column experiments to study adsorption, Desorption and transport of carbofuran within two acidic soils. Chemosphere. 2012; 88(1): 106-120.
- Arnaud B, Richard C, Michel S. A comparison of five pesticides adsorption and Desorption processes in thirteen contrasting field soils. Chemosphere. 2005; 61(5): 668-676.
- Chunxian W, Jin-Jun W, Su-Zhi Z, Zhong-Ming Z. Adsorption and Desorption of Methiopyrsulfuron in Soils. Pedosphere. 2011; 21(3): 380-388.
- Chunxian W, Suzhi Z, Guo N, Zhongming Z, Jinjun W. Adsorption and Desorption of herbicide monosulfuron-ester in Chinese soils. J Environ Sci. 2011; 23(9): 1524-1532.
- Christine MFB, Josette MF. Adsorption-desorption and leaching of phenylurea herbicides on soils. Talanta. 1996; 43(10): 1793-1802.
|