Vegetable oils are perceived to be alternatives to mineral oils for lubricant base oils because of certain inherent technical properties and their biodegradability. Vegetables oils with high oleic contents are considered to be the best alternative to substitute conventional mineral oil-based lubricating oils and synthetic esters. Most of the vegetable oils have high contents of unsaturated fatty acid and can be converted into epoxy fatty acid by conventional epoxidation. Nowadays epoxidized vegetable oil is having great concern as they obtain from sustainable, renewable natural resource and are environmental friendly. This project work mainly reveals about extraction of oil from dry pongamia seeds and study of its composition, physico-chemical properties and lastly its modification into triesters to improve the oxidation and cold flow behavior by epoxidation process. To produce epoxidized pongamia oil, the epoxidation was carried out by conventional chemistry at 50°C, speed of 550 RPM, and atmospheric pressure for about 10 h. An excess amount of hydrogen peroxide was necessary in the reaction to achieve high reaction conversion. By using this epoxidized oil the viscosity, viscosity index, flash point seems to be better for lubricant application.
INTRODUCTION |
Vegetable oil |
| At present, majority of the chemical products are produced from petrochemical feedstocks. Vegetable oils are a natural
and renewable product in an age of declining fossil oil reserves and growing demand from the energy and material
sectors. They have low volatility due to the high molecular weight of triacylglycerol molecule, excellent lubricity, and
favourable viscosity-temperature characteristics (narrow range of viscosity changes with temperature). In addition,
vegetable oils have high solubilizing power for polar contaminants and additive molecules than the mineral base oils.
Polar ester groups are able to adhere to metal surfaces, and therefore, possess good boundary lubrication properties.
Vegetable oils with high oleic content are considered to be the potential alternatives to conventional mineral oil based
lubricating oils and synthetic esters . |
| Vegetable oils are preferred over synthetic fluids because they are renewable resources and cheaper. Furthermore,
vegetable oils lubricants are biodegradable and non-toxic, unlike conventional mineral based oils. When vegetable oils
are used as base stocks for lubricants, they exhibit good lubricity and high viscosity index. |
| However vegetable oil has some drawbacks of poor corrosion resistance, poor thermal and oxidative stability which
confines their use as lubricants. Low temperature study has also shown that most vegetables oils undergo cloudiness,
precipitation, poor flow, and solidification at -100C upon long-term exposure to cold temperature in sharp contrast to
mineral oil based fluids. These physical and chemical properties can be improved either using genetically modified oils or chemically
modified oil with suitable combination of additives .Plant species, which have 30% or more fixed oil in their seeds or
kernel, have been identified. Traditionally the collection and selling of tree oilseeds was generally carried out by poor
people for use as fuel for lightening. Presently there is an extended use of these oils in soaps, shampoos, varnishes, bio
lubricants, candles, cosmetics, biodiesel, etc. However, the current utilization of non-edible oilseeds is very low. |
| The unsaturation present in vegetable oils can be chemically modified to a value added product by a complicated
reaction called „Epoxidation‟. Epoxidation is another convenient method to improve the poor thermo-oxidative stability caused by the presence of unsaturated double bonds in vegetable oils. Thus epoxidized vegetable oils are the promising
intermediates for the utilisation of vegetable oils. |
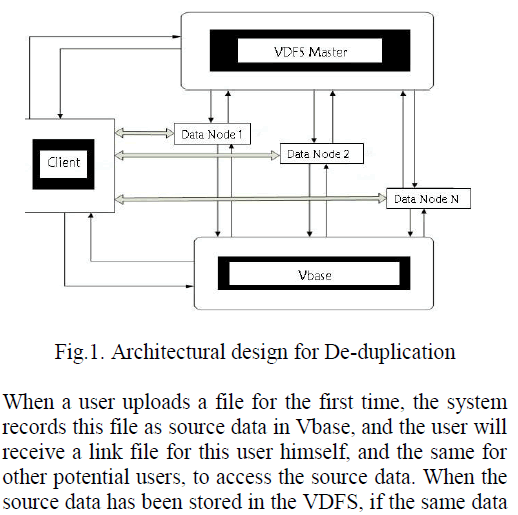
| Vegetable oils are predominantly the triglyceride molecules. Triglycerides are also known by the name triacylglycerol
(TAG). In vegetable oil glycerol molecule is attached to the three fatty acid chains of unsaturated and saturated fatty
acids. The saturated fatty acids contain only single bond between two carbon-carbon atoms while the unsaturated fatty
acids contain many double or triple bond between two carbon atoms. A generalized structure of triglyceride is shown in
the following figure. |
 |
BIOLUBRICANT |
| Lubricants act as an antifriction media, easing smoother working, cutting down the risks of undesirable frequently
encountered failures and maintaining authentic machine operations. Lubricants are essential for lubrication, heat
transfer, power transmission and corrosion protection in machinery in general. Lubricants consist of a mixture of base
oils with various additives, which can act to improve some of their properties. The basestocks may be of petroleum,
vegetable or synthetic nature. Mineral oils are derived from petroleum and represent about 95% of the lubricants
market in the world. The final composition of the lubricant may have 60-99% of base oils and the remaining as additive,
depending on the desired performance. The chief characteristic [17] of a lubricant is its viscosity, since this is what
prevents contact between the bearing surfaces. Other significant elements used to select a lubricant are compatibility,
toxicity, chemical stability, corrosiveness, flammability, environmental effects, availability, temperature stability and
price. |
| The fact is that, this oil may not be longer available; industries have been exploring for a cheap, renewable source of
lubricant. Vegetable oils are perceived to be alternatives to petroleum oils for lubricant formulations because of certain
inherent technical properties and their ability towards biodegradability. Due to environmental concerns, future
lubricants must be ecofriendly and come from a renewable source. Formulations made from vegetable based oils
together with corresponding additives are usually coined “Biolubricants”. Biolubricants have been the most anticipating
as they have useful physicochemical properties, but they are also have unsuitable properties that make petroleum based
lubricants the evident option. |
| The replacement of petroleum based lubricants with lubricants derived from vegetable oils is a very worthy and
alluring objective. The lessening of dependence on nonrenewable resources, reduction of greenhouse gases and
increase in markets for agricultural products these all outcomes are attractive to many countries. Bio lubricant is a
product, other than food or feed, substantially composed of certain biological products agricultural materials or forestry
materials. The product is used in place of a petroleum based lubricant. |
| It has necessitated the governments, research communities, and private organizations around the world to look for
alternative and renewable sources of energy due to the depletion of petroleum reserves, increase in energy demands,
unpredictability of fossil oil production, and increased concerns of rising greenhouse gas emissions. To date, many
alternatives have been researched and demonstrated but only a few have been proven to be practically feasible in terms of availability, economics, public and environmental safety, and simplicity of use. One such possible alternative is
biodiesel from vegetable oils, used at 100% or blended with diesel fuel for compression-ignition type engines. Soya
bean oil is relatively cheap when compared to mineral oils and other vegetable oils. In addition, soya bean oil is safer
and environmentally friendly. Crankcase lubricants are either petroleum based or mineral oil based. Prices of these
synthetic oils are significantly higher compared to vegetable oil-based lubricants. Although soya bean oil and its
derivatives like methyl esters have many properties that are conducive as crankcase oils, in depth engine studies on the
functionality of these oil forms are limited. |
MATERIALS AND METHODS |
Oil Extraction |
| A Pongamia pinnata seed (Pongamia glabra) was brought from a local fodder shop in Vellore, India. The seeds were
grinded into fine particles and by use of n-hexane solvent. All other chemicals and reagents were utilized from Poonga
Biotech Laboratory, Chennai. Extraction is one of the key processing steps in recovering oils contained in seeds.
Mechanical pressing is the simplest method of extraction, however, needs no extraction medium. It has been
traditionally applied to the extraction of oils from oil seeds; the only equipment needed is a hydraulic press. |
| Oil extraction can be done with or without seed coat; for Pongamia utilization of a mechanical de hulling system (to
remove the seed coat) can increase oil yield by 10 percent. |
Working of the apparatus |
| Soxhlet extraction methods enhances the efficiency up to 99%. In solvent extraction method generally n-hexane or nheptane
solvent is employed .The seeds were grinded into fine particles and 60gms of the grinded seed was taken and a
thimble was made. A soxhlet apparatus is only required when the desired compound has limited solubility in a solvent,
and the impurity is insoluble in that solvent. After extraction of pongamia oil, solvent is removed by rotator evaporator
at 500 C, yielding the extracted compound that is pongamia oil. A Soxhlet extractor is a piece of laboratory apparatus
invented in 1879 by Franz Von Soxhlet. |
| Typically, a Soxhlet extraction is only required where the desired compound has a limited solubility in a solvent, and
the impurity is insoluble in that solvent. Normally a solid material containing some of the desired compound is placed
inside a thimble made from thick filter paper, which is loaded into the main chamber of the Soxhlet extractor. The
Soxhlet extractor is placed onto a flask containing the extraction solvent. The Soxhlet is then equipped with a
condenser. The solvent is then heated to reflux. The solvent vapour travels up a distillation arm and floods into the
chamber housing the thimble of solid. The condenser ensures that any solvent vapours cools, and drips back down into
the chamber housing the solid material. The chamber containing the solid material is slowly filled with warm solvent.
Some of the desired compounds then get dissolved in the warm solvent. When Soxhlet chamber is almost full, the
chamber is automatically emptied by a siphon side arm, with the solvent running back down to the distillation flask.
This cycle is allowed to repeat several times within 8hrs of extraction. During each cycle, a portion of the non-volatile
compound dissolves in the solvent. After many cycles the desired compound is concentrated in the distillation flask.
After extraction the solvent is removed, typically by means of a rotary evaporator at 40-50°C, yielding the extracted
compound i.e. oil. The non-soluble portion of the extracted solid remains in the thimble, which is discarded. The
development of new, efficient, and environmentally benign pathways, which can lead to new value added products, is
still an area with high potential. This strategy can decrease our dependence on non-renewable, and therefore limited,
resources such as mineral oil. Vegetable oils as biolubricants are preferred because they are biodegradable and nontoxic,
unlike conventional mineral-based oils. Vegetable oils have different properties than mineral oils due to different
chemical structures. They have very low volatility due to the higher molecular weight of the triacylglycerol molecule
and a narrow range of viscosity changes with temperature. Superior anticorrosion properties of vegetable oils result
from their high affinity for metal surfaces.. Polar ester groups are able to adhere to metal surfaces, and, therefore,
possess good boundary lubrication properties. In addition, vegetable oils have high solubilizing power for polar
contaminants and additive molecules.. These characteristics are disadvantages of vegetable oils, in sharp contrast to
mineral oil-based fluids. The oil is used by common people due to its low cost and easy availability. The fresh extracted oil is yellowish orange to brown, and rapidly darkens on storage. It has a disagreeable odour and bitter taste. Solvent
extraction of expelled cake yields better quality light yellow oil. |
Epoxidation |
| This method gives partially saturated derivatives with oxygen. Pongamia seeds are good source of oleic acid as its
percentage is 51.59 and are thermally stable than polyunsaturated fats, and therefore are highly desired component in
vegetable oils for lubricant applications. Pongamia can be successfully propagated through seeds and cuttings.
Modification of Pongamia oil through chemical processing to improve oxidation stability and low temperature fluidity
is made possible by combining it with chemical additive and hence such chemical modification made to improve the
cold flow behavior of vegetable oils for the use as bio lubricant base oil. Chemical modification of vegetable oils is an
attractive way to solve these problems and to obtain valuable commercial products from renewable raw materials. |
Process of Epoxidation |
| It is the most widely used process of epoxidation. For safety point of view these epoxidation are usually carried out
using peracids formed in-situ, by reacting a carboxylic acid with concentrated hydrogen peroxide. This process
performs industrially on large scale. |
| At first, Soybean oil and formic acid were poured to a glass and mechanically stirred and the temperature controlled
then, the glass was fixed by a metal clamp in a Benmary (water bath) with water temperature of 50˚C± 2 and speed of
550 RPM. To start the epoxidation, Hydrogen Peroxide solution (30 %) was gradually charged into the mixture during
the first 5 h of reaction. mole ratios of carbon double bonds to Hydrogen Peroxide (C=C:H2O2) were used; 1:1.7 After
charging H2O2 was completed, the reaction continued by mixing and controlling the temperature at 50˚C for a further
5 h. After that, the mixture was cooled down and neutralized by water. Diethyl ether was used to enhance the
separation of the oil product from water phase. The final product was dried out by heating less than 50˚C. Three
replications were performed concurrently. The production procedure of ESO is shown in Figure 2.the double bonds are
one of the active sites that can react with functional groups |
 |
 |
 |
Epoxidized Vegetable Oil |
| Epoxidized vegetable oil has unlimited and bright future prospects as it is found to be potential and renewable bio
based material. Increasing the concern about environmental issues and limiting supply of petroleum and fossil fuel
again adds the importance to epoxidized vegetable oil. Epoxidized vegetable oil possesses epoxy ring in their backbone
chain and produces flexibility and elasticity when it is treated with thermoplastic or thermosetting polymer along with
suitable curing agent. Because of this special kind of application EVO can easily replace the phthalates which are
petroleum based. Looking forward this it is expected that EVO oil has a great future. |
RESULTS |
The Physico-Chemical Properties Of Oil |
| The physicochemical properties of pongamia oil are shown in the table given below |
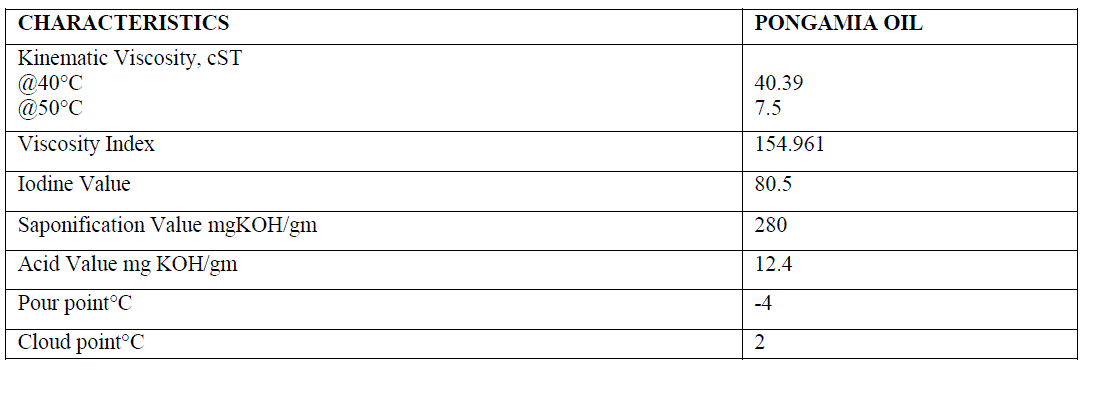 |
The Physico-Chemical Properties Of Epoxidized Oil |
| The physicochemical properties of Epoxidized oil are shown in the table given below |
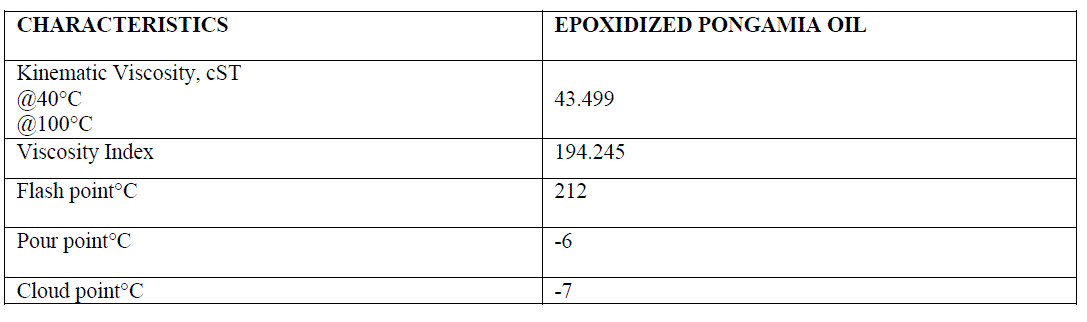 |
The Physico-Chemical Properties Of Mineral Oil Lubricant |
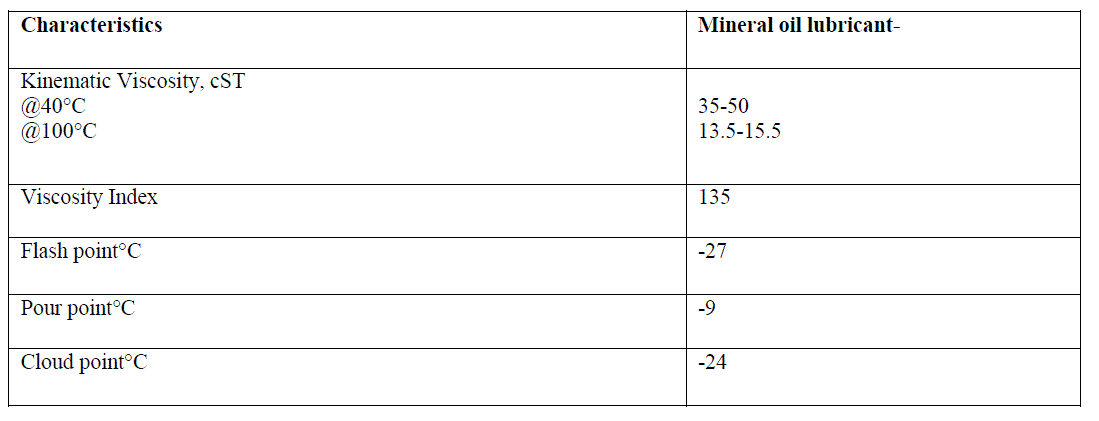
| The physicochemical properties of mineral oil lubricant are shown in the table given below |
FTIR Analysis |
 |
| The structures of the synthesized compounds were confirmed were confirmed using Fourier-Transformed Infrared
(FTIR) spectroscopy. The characteristic signals in the FTIR spectrum of epoxidized oleic acid (1) at 830, 845cm-1
correspond to quaternary carbons of the oxirane ring and the signals at 2987 and 2865 cm-1 correspond to aliphatic
carbons in the molecules. In mono-ester product the bands at 1738 and 1710 cm-1 due to C=O stretching vibrations of
ester and carboxylic acid moieties confirm the success of oxirane ring opening step. Furthermore, the most
characteristic evidence confirms trimester formation was the disappearance of OH stretching vibration around 3400
cm-1. |
CONCLUSION |
| The potential utility of epoxidized vegetable oil has begun to be realized in industrial applications with increasing the
concern of research in aspirants to develop value added products from the available plant oils. Vegetable oil could be
epoxidized successfully by peroxy acid generated „in-situ‟ by reacting formic or acetic acid with hydrogen peroxide at
isothermal temperature condition. The epoxidation can be confirmed by iodine value. In-situ epoxidation of vegetable oil is more convenient and economically viable method for large scale epoxidation which shows utility especially in
plasticizer and stabilizer used in polymers. In the present study, several basic trends were observed. The prepared
compounds exhibited the favorable cold-flow characteristics. The presence of branching group at the head of the
molecule will make it more effectively in disruption crystalline formation at reduced temperatures. These products can
be efficiently utilized for bio based industrial materials, such as bio lubricants. Contaminated environment is expensive.
Conventional mineral oil based lubricants are extremely harmful for the biosphere when they get into the environment.
Due to poor degradability mineral oils remain in the ecosystem for a long time. Even in case of high dilution the effect
will be fatal (ecotoxicological effect). Higher amount will be required for elimination of contaminated ecosystem
clearly. Edible oils in use in developed nations such as USA and European nations but in developing countries the
production of edible oils are not sufficient. In a country like India, there are many plant species whose seeds remain
unutilized and underutilized have been tried for biodiesel production. Non-edible oil seeds are the potential feedstock
for production of bio lubricant in India. |
References |
- Dinda S, Patwardhan AV, Goud VV &Pradhan NC (2008), Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquidinorganic acids‟ Bioresource Technology, vol.99, no.9, pp. 3737-3744.
- Meyer(2008)BiobasedEpoxidized Vegetable Oils And Its Greener Epoxy Blends: A Review, 10.1080/03602559.2010.512338
- Cai C, Dai H, Chen R, Su, C, Xu X, Zhang , S and Yang L 2008, “Studies on the kinetics of insitu- epoxidation of vegetable oil” , EuropeanJournal of Lipid Science and Technology vol.110, no.4, pp.341-346.
- RusksharAlam “Studies on properties of karanja oil” Department Of ChemistryNational Institute Of Technology, Rourkela.
- Jai Liakum(2010) “ Synthesis Of Vegetable Oil Based Polyether Polyols Via Epoxidation Followed By Ring-Opening Reaction”
- Taydesaurabh(2013) “Epoxidation of vegetable oils: A Review” International journal of advanced engineering technology, E-ISSN 0976-3945
- Sujay U. Mahajan(2013) “Synthesis and Characterization of Chemically Modified Epoxidized Mustard Oil for Biolubricant Properties”International Journal of applied Engineering Research;ISSN 0973-4562.
- P. Saithai, J. Lecomte, E. Dubreucq (2013), V. Tanrattanakul, “Effects of different epoxidation methods of soybean oil on the characteristics ofacrylatedepoxidized soybean oil-co-poly(methyl methacrylate) copolymer” 10.3144/expresspolymlett.2013.89
- F. Murilo T. Luna(2011) “Assessment of biodegradability and oxidation stability of mineral, vegetable and synthetic oil samples” IndustrialCrops and Products 33 (2011) 579–583.
- KRIAPAT CHEENKACHOR, “Development of engine oil using palm oil as a base stock for four stroke engine”, Energy 35(2010) 2552E2556
- Chen-Ching Ting and Chain-ChihChen(2013) “Viscosity and working efficiency analysis of soybean oil based bio-lubricants”, ElsevierMeasurement 44 (2011) 1337–1341.
- S. Syahrullail, B.M.Zubil, C.S.N. Azwadi,(2011) “Experimental evaluation of palm oil as lubricant in cold forward extrusion process” International Journal of Mechanical Sciences (Impact Factor: 1.61). 07/2011; 53(7):549-555
- KourooshSaremi (2012) “Epoxidation Of Soyabean Oil” Annals of Biological Research, , 3 (9):4254-4258
- Findley, T.W., Swern, D. and Scanlan, J.T., “Epoxidation of Unsaturated Fatty Materials with Peracetic Acid in Glacial Acetic Acid Solution”, Journal of the American Chemical Society, 67, 412-414, 1945.
- Goering CE, Schwab AW, Daugherty MJ, Pryde EH, Heakin AJ(2011), “Fuel properties of eleven vegetable oils”, Trans. ASAE1982;1472±1483.
|