Keywords
|
| adsorption, refrigeration cycle, refrigerator, solar energy |
INTRODUCTION
|
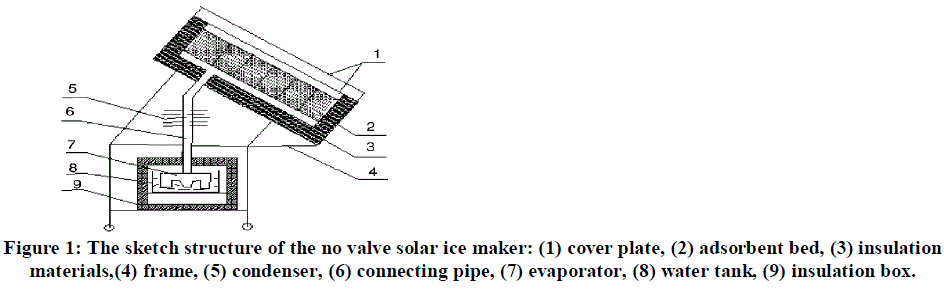
| The major attraction of solid adsorption refrigeration technology for cold production is that it can be powered entirely or partly by low grade energy such as solar energy, bio-energy etc. Its working fluids satisfy the Montreal protocol on ozone layer depletion and the Kyoto protocol on global warming. Furthermore, solar powered refrigeration based on adsorption cycle is simple, quiet in operation and adaptable to small, medium or large systems. Adsorption systems are invented with different focus. For one bed machine, simple structure and low cost are pursued. Several multi-bed systems are suggested to provide steady refrigeration and higher energy performance. One bed adsorption refrigerator is advantageous in simple structure and low initial cost. It can be used in applications when continuous cooling or higher cooling capacity is not required. Adsorption refrigeration system uses solid adsorbent beds to adsorb and desorb a refrigerant to obtain cooling effect. These solid adsorbent beds adsorb and desorb a refrigerant vapor in response to changes in the temperature of the adsorbent. Here adsorbent is an Activated carbon and the refrigerant used is methanol. The basic adsorption refrigeration system, commonly referred to as the adsorption heat pump loop, or an adsorption refrigeration circuit, it consists of four main components: a solid adsorbent bed, a condenser, and an evaporator ice-box. The solid adsorbent bed desorbs refrigerant when heated and adsorb refrigerant vapor when cooled. In this manner, the bed can be used as a thermal compressor to drive the refrigerant around the system to heat or cool a heat transfer fluid or to provide space heating or cooling. Thus in this system bed (of activated carbon) acts as compressor so as to drive refrigerant (methanol) similar to compressor in basic refrigerator. The refrigerant is desorbed from the bed as it is heated to drive the refrigerant out of the bed and the refrigerant vapor is conveyed to a condenser. In the condenser, the refrigerant vapor is cooled and condensed to liquid. The low pressure condensate passes to an evaporator where the low pressure condensate is heat exchanged with the process stream or space to be conditioned to vaporize the condensate. When further heating no longer produces desorbed refrigerant from the adsorbent bed, the bed is isolated and allowed to return to the adsorption conditions. When the adsorption conditions are established in the bed, the refrigerant vapor from the evaporator is reintroduced to the bed to complete the cycle. For the circulation of methanol in the system the whole system should be vaccumised. Figure 1 shows schematic layout of a adsorption refrigeration system running on solar enegry. The solar refrigerator consists of an adsorbent bed (2), a condenser (5), an evaporator (7), water tank (8), insulation box (9) as well as connecting pipes. For this system, there are no any reservoirs, connecting valves and throttling valve, the structure of the system is very simple. The working principle of this no valve solar refrigerator is described as follows. |
| On a sunny day, the adsorbent bed absorbs solar radiation energy, which raises the temperature of adsorbent bed as well as the pressure of methanol in adsorbent bed. When the temperature of adsorbent reaches the desorption temperature, the refrigerant begins to evaporate and desorb from the bed. The desorbed refrigerant vapor will be condensed into liquid via the condenser and flows into the evaporator directly; this desorption process lasts until the temperature of adsorbent reaches the maximum desorption temperature. During night, when the temperature of the adsorbent bed reduces, the refrigerant vapor from the evaporator gets adsorbent back in the bed. During this adsorption process, the cooling effect is released from refrigerant evaporation, and the ice is formed in the water tank placed inside thermal insulated water box. |
 |
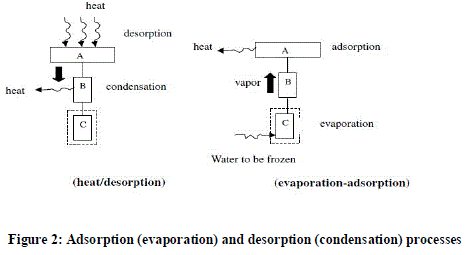
| Likely in a vapour compression system the adsorption refrigeration system also consists of a compressor, a condenser, and an evaporator but no throttle valve is used. However, in this system the compressor is replaced by a thermal compressor which is operated by heat instead of a mechanical energy. The vaporized refrigerant is adsorbed in the pores of the adsorbent in the reaction chamber i.e. adsorbent bed. Thus the operation of the adsorption cooling system depends on adsorption/desorption characteristics of the particular adsorbent/refrigerant pair. Due to the loading of the adsorbent, the thermal compressor is operated intermittently. |
II. ADSORPTION REFRIGERATION CYCLE
|
| Adsorption refrigeration system uses solid adsorbent beds to adsorb and desorb a refrigerant to obtain cooling effect. These solid adsorbent beds adsorb and desorb a refrigerant vapor in response to changes in the temperature of the adsorbent. The basic adsorption refrigeration system, commonly referred to as the adsorption heat pump loop, or an adsorption refrigeration circuit, usually consists of four main components: a solid adsorbent bed, a condenser, an expansion valve and an evaporator. The solid adsorbent bed desorbs refrigerant when heated and adsorb refrigerant vapor when cooled. |
 |
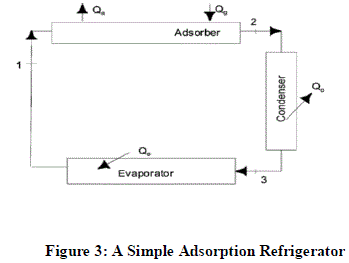 |
| Where, Qg = The quantity of energy used to generate refrigerant vapour from the adsorbent during the generation process, Qa = The quantity of heat released by adsorbent, Qe = The quantity of heat transferred in the evaporator during refrigeration process, Qc = The quantity of heat dissipated by the hot refrigerant vapour to change to liquid form |
III ANALYSIS OF ADSORPTION REFRIGERATION CYCLE
|
 |
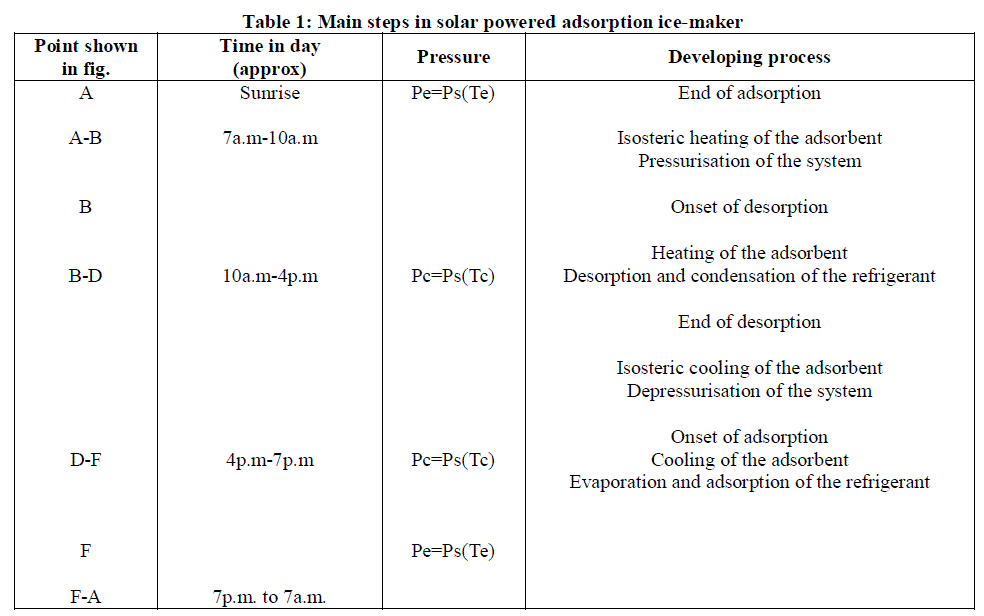
| The cycle for adsorption refrigeration of AC-methanol can explain by Clapeyron diagram in (in P versus 1) /T. |
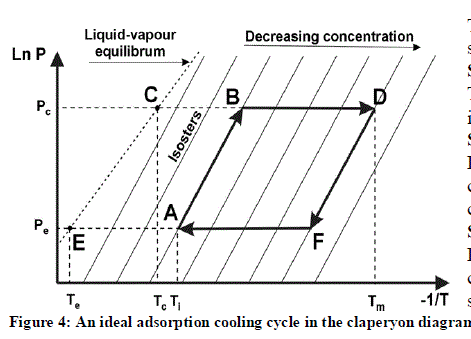 |
| The cycle is explained in detail in (Buchter F., 2001). We can summarize it in four stages: Step 1: Isosteric heating (A→B): The system temperature and pressure increase due to solar irradiance. Step 2: Desorption + condensation (B→D): Desorption of the methanol vapours contained in the activated carbon bed; condensation of the methanol steam in the condenser. Step 3: Isosteric cooling (D→F): Decrease of the period of sunshine; cooling of the activated carbon; decrease of the pressure and the temperature in the system. |
| Step 4: Adsorption + evaporation (F→A): |
| Evaporation of methanol contained in the evaporator; cooling of the cold cabinet; production of ice in the evaporator; re-adsorption of methanol steam by the activated carbon. |
 |
IV. MATHEMATICAL MODEL OF THE SYSTEM
|
 |
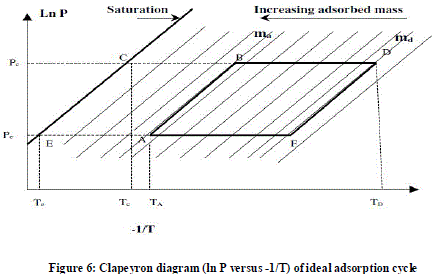 |
| Tbdes = TEMPERATURE BEFORE DESORPTION |
| This temperature is taken before desorption process starts i.e. during morning session measured at adsorbent bed. |
| Tades = TEMPERATURE AFTER DESORPTION |
| This temperature is taken after desorption completes i.e at the end of day during evening session at adsorbent bed. |
| Ta = TEMPERATURE AT POINT A |
| This temperature is taken before afternoon session at the start of isosteric heating at adsorbent bed. |
| Tb = TEMPERATURE AT POINT B |
| This temperature is taken at afternoon time of the day and at the end of isosteric heating and start of desorption process at adsorbent bed. |
| Td = TEMPERATURE AT POINT D |
| This temperature is taken during evening session after desorption completes at adsorbent bed. |
| Tcc = TEMPERATURE OF CONDENSATE |
| This is temp of condensate measured by measuring water/ice temperature at ice box during early morning session. |
| Tee = TEMPERATURE OF EVAPORATE |
| This is temperature of evaporate measured by measuring water/ice temperature at ice box during early morning session. |
| By Clapeyron theory the mass of methanol at point A and D is Let, Mm = 1.3575 KG, mass of total methanol in system. Mma = Mm*(75/100), mass of methanol at point A = 1.3575*0.75 = 1.018125 KG Mmd = Mm*(25/100), mass of methanol at point D = 1.3575*0.25 = 0.339375 KG |
V. OBSERVATIONS
|
 |
VI. EXPERIMENTAL INVESTIGATIONS
|
| As the system is a pilot project, experiments are carried out so as to check the exact working of the system. Following are some of the analysis made on the system. |
VI. 1- VARIATION OF TEMPERATURE OF ADSORBENT BED WITH TIME:
|
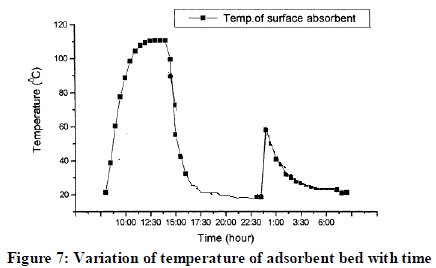
| The graph shown below is expected behavior of adsorbent bed. It is variation of temperature in the adsorbent bed because of incident solar radiations. Temperature in bed is directly proportional to the incident solar radiation. Effective solar radiations are available during period of 10 am to 3pm thus the temperature in bed during this period reaches to 100 ºC to 120 ºC. After 3 pm the temperature in bed gets lower and lower. Till 5.30 pm the temperature lowers to 20dgree c and desorption continues till that time. After this, temperature remains almost constant till 10.30 pm, now the adsorption of methanol starts. As adsorbent bed adsorbs vapours of methanol thus the temperature of adsorbent bed increases up to 60 ºC, approximately at midnight temperature is about 60 ºC – 70 ºC. After this the temperature again starts decreasing and exothermic reaction carry on. And now the bed will attain temperature of surrounding i.e. 25 °C. |
VI. 2- VARIATION OF TEMPERATUTE OF WATER IN ICE-BOX WITH RESPECT TO TIME
|
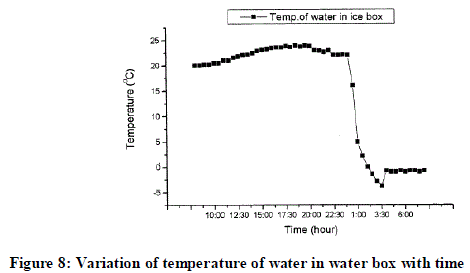
| The graph shown below is the expected graph of variation of temperature of water in ice-box with respect to time. |
 |
 |
VII. ANALYSIS OF OBSERVATIONS
|
| The graph shows that during desorption process the temperature of water is 20 ºC, it remains constant till the desorption process ends. Desorption process has duration of about 7-8 hours. It ends approximately at 7 pm. As the desorption ceases the adsorption process will start. After a particular timing the temperature of water decreases rapidly, that timing is critical timing of approximately about 11.30p.m. During this process methanol absorbs heat of water and thus methanol will evaporate and evaporated methanol will be adsorbed by AC. Thus ice is formed. And temperature of this ice will be -5 ºC at about 3.30 am. After 3.30 am temperature of water increases slightly to 0 ºC. It remains constant till approximately 7am. Now the ice produced can be removed from the box. |
 |
VIII.CONCLUSION
|
| It is possible to consider that adsorption systems can be alternative to reduce the CO2 emissions and electricity demand when they driven by waste heat or solar energy. Although, for a broader utilization the researches should continue aiming for improvements in heat transfer, reductions of manufacturing costs and for the formulation of new adsorbent compounds with enhanced adsorption capacity and improved heat and mass transfer properties. Because adsorption systems are generally poor thermal machines. The performance of adsorption systems depend strongly on the adsorption and condensation temperatures. The following conclusions may be drawn from the foregoing solar powered solid adsorption ice-maker studies:- -A solar powered solid adsorption icemaker using an activated carbon/methanol adsorbent pair has been successfully designed, constructed and tested -The condenser and evaporator must necessarily be close to each other and to the collector since the system operates at low pressure, thus they are located directly under the collector such that the refrigerant flows into them by gravity. -The adsorption bed (generator) is the heart of the system and it has the greatest effect on the performance of the system. A good design of the generator leads to smooth operation and better results, so more attention must be go to the design influence on the performance of the system. |
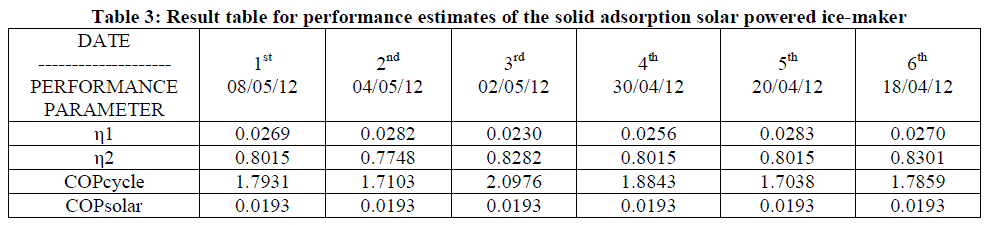
| -The ice produced can be used for various purposes such as in hospitals or for domestic use. -The adsorption /desorption tests for activated carbon/methanol pair showed that there must be sufficient time to get the highest desorption of methanol, and the optimum time for that was found to be 5-10 hours. The generation temperature must be over 100 °C in order to generate higher volume of methanol from activated carbon. -COP of the system is 0.12, which is comparatively low but as the system works on solar energy it is eco-friendly system. -From this study, one can conclude that the possibility of using nonpolluting materials and to save the energy involved in this sector are obviously the most important characteristics but simplicity, low maintenance, and the absence of noisy components are also very important features that make this type of system suitable for numerous other applications i.e. future scope of the project is such as air-conditioning in cars or food transportations or solar cooling with the use of: multi-bed systems. |
References
|
- M. Pons and J. J. Guillemiont; Study of solar-powered, solid-adsorption ice maker, Trans. ASME, Solar Energy Eng. 108 (4) 332–337 (1986).
- R.E. Critoph, Performance limitations of adsorption cycles for solar cooling, Solar Energy 41, 21–31 (1988).
- Critoph. R. E., Limitations of adsorption cycles for solar cooling. Solar Energy, 41(1), 23- 31 (1988).
- O.S. Headley, A. F. Kothdiwala and I.A. Mcdoom; Charcoal-methanol adsorption refrigerator powered by a compound parabolic concentrating solar collector; Solar Energy, 53, 191-197 (1994)
- Wang, R.Z., J.P.Jia, Y.H. Zhu, Y.Teng, J.Y. Wu and J. Cheng; Study on a new solid adsorption refrigeration pair: active carbon fibre-methanol; ASME Journal Solar Energy Engg, 119, 214-219 (1997).
- R.Z. Wang, Y.X. Xu, J.Y. Wu and W. Wang; Experiments on heat regenerative adsorption refrigerator and heat pump; International journal of energy research 22, 935-941 (1998).
- Li Zhongfu and K. Sumathy; A solar-powered ice-maker with the solid adsorption pair of activated carbon and methanol; International Journal of Energy Research, 23, 517-527 (1999).
- R.Z. Wang, M. Li, J.Y. Wu; An energy efficient hybrid system of solar powered water heater and adsorption ice maker; Solar Energy 68 (2) 189–195 (2000).
- R. Z. Wang; Adsorption Refrigeration Research in Shanghai Jiao Tong University; Renewable and Sustainable Energy Reviews 5, 1-37 (2001).
- A.O. Dieng and R.Z. Wang; Literature review on solar adsorption technologies for ice making and air conditioning purposes and recent developments in solar technology; Renewable and sustainable energy reviews 5, 313-342 (2001).
- M. Li, R.Z. Wang, Y.X. Xu, J.Y. Wu, A.O. Dieng; Experimental study on dynamic performance analysis of a flat plate solar solid-adsorption refrigeration for ice maker; Renewable Energy 27, 211–221 (2002).
- M. Li, R.Z. Wang; A study of the effects of collector and environment parameters on the performance of a solar powered solid adsorption refrigerator; Renewable Energy 27, 369–382 (2002).
- M. Li, R.Z. Wang; Experimental of a solar flat plate hybrid system with heating and cooling; Applied Thermal Engineering, 1445–1454 (2002).
- M. Li, R.Z. Wang, Y.X. Xu; Experimental study on dynamic performance analysis of a flat plate solar solid adsorption refrigeration for ice maker; Renewable Energy 27, 211–221 (2002).
- M. Li, R.Z. Wang; A study of the effects of collector and environment parameters on the performance of a solar powered solid adsorption refrigerator; Renewable Energy 27, 369–382 (2002).
- M. Li, R.Z. Wang; Heat and mass transfer in a flat plate solar solid adsorption; Renewable Energy 28, 613–622 (2003).
- L.W. Wang, R.Z. Wang, J.Y. Wu and K. Wang; Compound adsorbent for adsorption ice maker on fishing boats; International journal of refrigeration 27, 401-408 (2004).
- M. Li a, H.B. Huang b, R.Z. Wang, L.L. Wang, W.M. Yang and W.D. Cai; Study on intermittent refrigeration phenomenon for solar solid adsorption refrigeration; January 2005
- R. Z. Wang and R. G. Oliveira; Adsorption Refrigeration- An efficient way to make good use of waste heat and solar energy; International sorption heat pump conference, June 22-24, 2005; Denver CO USA, ISHPC- 101 K- 2005.
- Zhaohui Qi; Study on hybrid system of solar powered water heater and adsorption ice maker; International journal of architectural science,Volume 6, No. 4, 168-172, 2005.
- Activated Carbon and Related Technology; Cameron Carbon Incorporated 2006.
- Li Yong and Ruzhu Z. Wang; Adsorption Refrigeration: A Survey of Novel Technologies; Recent Patents on Engineering 2007, 1, 1-21.
- G. Halder and S. C. Sarkar; The cooling effect by an adsorption- desorption refrigeration cycle; Journal of Energy in Southern Africa, Vol 18 No 2, May 2007.
- W. Chekirou, N. Boukheit and T. Kerbache; Numerical modeling of combined heat and mass transfer in a tubular adsorber of a solid adsorption solar refrigerator; Revue des Energies RenouvelablesVOl. 10 Nº 3 (2007) 367-379.
- Kai Wang and Edward A. Vineyard; New opportunities for Solar Adsorption Refrigeration; a article in ASHRAE Journal, September 2011.
- PeerapongThumautok, WipawadeeWongsuwan and TanongkiatKiatsiriroat; Performance analysis of a solar adsorption heating and cooling systems; Chiang Mai University (CMU), Thailand, 50200.
- Piero M. Armenante; Adsorption; NJIT.
- Robert D. Goodwin; Methanol thermodynamic properties from 176 to 673 K at pressures to 700 bar; Thermophysics division, National Engineering Laboratory, National Bureau of Standards, Boulder, Colarado 80303, June 1987.
> |