e-ISSN: 2321-6182 p-ISSN: 2347-2332
e-ISSN: 2321-6182 p-ISSN: 2347-2332
Nishi Saxena*, and Ameeta Argal
Rajeev Gandhi College of Pharmacy, Bhopal, Madhya Pradesh, India
Received: 17/11/2013; Revised: 12/12/2013; Accepted: 14/12/2013
Visit for more related articles at Research & Reviews: Journal of Pharmacognosy and Phytochemistry
Boerhaavia diffusa L.root powder was studied for macroscopic, micromeretic & phytochemical parameters. It was coarse, light brown powder with very few foreign organic matters. Angle of repose, bulk density and tapped density showed passable flow property due to uneven shape and rough surface. The total ash, acid insoluble ash and water soluble ash values were within IP limit showing the purity of drug. Alcohol soluble and water soluble extractive values were found to be 18.6% & 23.2% w/w respectively. The phytochemical investigation showed the presence of carbohydrates, alkaloid, protein, terpenoid and flavanoid in both aqueous and alcoholic extract. The presence of these constituents is responsible for the wide medicinal use of Boerhaavia diffusa L. roots.
Boerhaavia diffusa L, angle of repose, extractive values
Herbal drugs are essential components of traditional medicine in several countries including China and India [1]. Boerhaavia diffusa L. (Nyctaginacea) is one such important herbal drug. It is an important medicinal plant much used in Ayurveda and Unani medicines and other traditional medicines in many parts of the world. The plant is a rich source of vitamins, minerals, protein and carbohydrate and contain a number of constituents like alkaloids, flavonoids, saponins and steroids. Its roots have been reported to contain alkaloids (punarnavine), rotenoids (boeravinones), flavonoids, amino acids, ligans (liriodendrons), ß sitosterols, and tetracosanoic, esacosanoic, stearic and ursolic acids. Traditionally, this plant has been extensively used in the treatment of dyspepsia, jaundice, enlargement of liver, abdominal pain and as antistress agent. It also has diuretic, anti-inflammatory, antidiabetic, antibacterial and anticancer properties [2,3]. The present work has been taken up for evaluating various parameters for identification and purity of drug.
The powder of roots of Boerhaavia diffusa L. was procured from Govt. emporium of crude drugs of Bhopal (M.P.). It was subjected to pharmacognostic, physicochemical and micromeritic studies. The crude powder was then macerated with alcohol for five days, filtered and concentrated to get alcoholic extract.
Macroscopic examination [4]
The morphological studies of Boerhaavia diffusa L. was done. The powder of Boerhaavia diffusa L. was coarse, light brown in colour with a bitter taste and characteristic odour.
Thin Layer chromatography [5]
Silica gel G coated plates were used. Punarnava roots were taken as reference standard. They were coarsely powdered, refluxed with methanol, filtered And concentrated. This was reference solution. Similarly the powder of test sample of Boerhaavia diffusa L. as treated to get the test solution. Both the solutions were applied to the silica gel plate. Mobile phase used was a mixture of 35 ml of chloroform, 6 ml of methanol and 1 ml of glacial acetic acid. The plate was developed with anisaldehyde solution. TLC of sample was compared with that of reference standard.
Micromeretic parameters [6]
Angle of repose
It was calculated by allowing 50 gm of powdered drug to flow though a funnel, such that the distance between the top of the pile and bottom of funnel is 6.4mm. The height and radius of the powder pile was noted and angle of repose, was calculated by
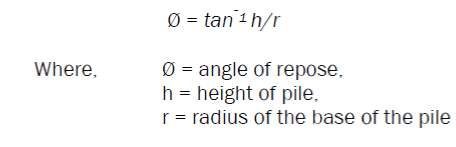
Bulk Density
50 gms of powder was taken in a bulk density apparatus, and the volume of powder (bulk volume) was noted. The bulk density was calculated by substituting the values in the formula:
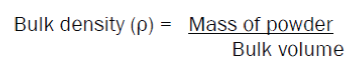
Tapped Density
50 gms of powder was taken in a bulk density apparatus, and the volume of powder (bulk volume) was noted. The apparatus was set for 100 tappings. The tappings were continued till concurrent readings were obtained. Final volume was noted as tapped volume. Tapped density was calculated by substituting the values in the formula:
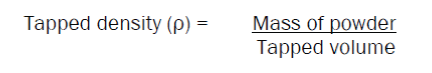
Physicochemical Evaluation [7,8]
Determination of foreign matter
The sample was checked visually for the presence of foreign organic matter. Very few foreign particle were found (Table 2).
Loss on drying
Five gm of powder was dried in oven at 105ºC and weighed
Determination of Ash value
Determination of total ash
2 gm of the crude drug was incinerated at about 450°C. It was cooled, weighed and total ash was calculated (Table 2).
Determination of acid insoluble ash
The total ash obtained above was boiled with 25ml of 2M HCl for 5 min. It was collected on a tared filter paper, dried and then weighed (Table 2).
Determination of water soluble ash
The total ash was boiled for 5 min. with 25ml of water, collected on a tared filter paper, dried and weighed. The weight of the insoluble matter was subtracted from the weight of the ash to get water soluble ash (Table 2).
Determination of solvent extractive value
Percentage of water soluble extract or alcohol soluble extract was determined with reference to air dried drug.
Determination of alcohol soluble extractive value
Five gm of drug powder was macerated with 100ml of ethanol for 24 hr. It was shaken frequently for first six hrs. It was then filtered. 25ml of the alcoholic extract was evaporated to dryness in a petri dish. It was further dried at 105ºC and weighed. Percentage of ethanol soluble extractive value was calculated with reference to air dried drug taken (Table 2).
Determination of water soluble extractive value
Five gm of drug powder was macerated with 100ml of chloroform water for 24 hr with shaking. After filtration solvent was evaporated to dryness (105ºC), weighed, & percentage of water soluble extractive value was calculated (Table 2).
The alcoholic and aqueous extracts of Boerhavia diffusa L. were subjected to various qualitative tests to reveal the presence or absence of common chemical constituents (Table 3).
The root powder of Boerhaavia diffusa L., was coarse, light brown in colour with a bitter taste and characteristic odour. TLC studies showed similar chromatogram as that of reference standard of Boerhaavia diffusa L [4]. This proves the identity of drug. The powder was evaluated for its micromeritic parameters. Angle of repose was found to be 39.69̡̉, bulk density was 0.34 and tapped density was 0.55. The powder shows passable [9] flow property due to uneven shape and rough surface. It showed the presence of foreign organic matter (0.2%), total ash (8.6% w/w), acid insoluble ash (2.4% w/w) and water soluble ash (4.8% w/w). It is within the guidelines of IP. This reveals the drug is pure. Alcohol soluble extractive and water soluble extractive values were found to be 18.6% & 23.2% w/w respectively. This shows the nature of the constituents present in crude drug [8]. As the percent yield is good its use can be economical. The phytochemical investigation showed the presence of carbohydrates, alkaloid, protein, terpenoid and flavanoid in both aqueous and alcoholic extract. The presence of these constituents is responsible for the wide medicinal use of Boerhaavia diffusa L. roots [10].
From the results of analysis it was found that the powdered drug of Boerhaavia diffusa L. roots procured from market is pure complying IP standards. Since it contains carbohydrates, alkaloid, protein, terpenoid and flavanoid it will be scientifically evaluated for various pharmacological activities as used by tribals and Ayurved.
The authors are thankful to AICTE, New Delhi for giving financial assistance for this work.