ISSN: 2320-0189
ISSN: 2320-0189
Biodiversity and Systematics Laboratory, Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, Punjab, India
Received date: 13 April 2014 Accepted date: 12 June 2015
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Many plant groups are known to deposit silicon within and between the cells and tissues in solid form creating amorphous structures commonly known as phytoliths or silica bodies. Phytoliths are inorganic amorphous oxides (SiO2) formed by the process of polymerization following uptake of monosilicic acid (H4SiO4) from the soil. Phytoliths are known to boost the growth and development of plants particularly during environmental onslaughts. They provide mechanical strength and rigidity to plant parts and serve as a defense system against predators, herbivores and fungal infestations as well as improve water balance, plant growth and yield, rates of photosynthesis, reproduction and reduce grain chaffness. Above all the characteristic shape and size of phytoliths are known to play significant role in taxonomic analysis of different plant groups. The present paper highlights the background on plant phytoliths, their distribution in plant kingdom, forms in the soil and the ones available to the plants, silica uptake mechanisms, its deposition and distribution within the plant body and their roles.
Phytoliths, Silica, Silica bodies, Grasses, Silicon
Phytoliths are produced as a result of biological and physical processes in certain plant groups and deposited as solid silica in intercellular or extracellular locations after absorbing silica in soluble form as monosilicilic acid (H4SiO4). The term “phytolith” was proposed by Ruprecht (1866) [1]. It is composed of two Greek words “phyton (φϋτον) = plant” and “lithos (λίθος) = stone” meaning plant stone. This term has been used to indicate all forms of mineralized substances deposited in higher plants, be they siliceous or calcareous in nature.
Although the term “phytolith” has been used for various types of mineral depositions, siliceous and calcareous particles often reveal substantial differences in their structure and taxonomic attributes. Therefore, it has been suggested that the term “phytolith” be used in a more restrictive sense to refer only to silicified incrustations.
Numerous other terms have also been used for silica bodies found in plants, such as “opal phytolith”, “plant opal” and “opaline silica”. The term opal has been used because of the color of the particles in reflected light. Phytoliths, like mineral opal deposited by geological processes, are not crystalline in structure. They are amorphous (non-crystalline) and have variable water content. Phytoliths are also put under a more generalized term, as “biogenic silica” or simply “bioliths”. These are all-inclusive terms for silicon found in plants and animals. They help to distinguish silica derived from living system from the silica of inorganic, mainly pedogenic origin. The plant does not use the silica for any of its metabolic processes and so deposits it as a siliceous gel within cavities in its own structure [2]. This gel, on increasing concentration and desiccation, gradually crystallizes into a solid, silt sized particle. Some of the enclosing cell material may be trapped inside the phytolith as it crystallizes [3, 4]. When the plant dies and decays, the phytoliths are released into the soil undergoing the same erosion, transport and depositional processes as other sedimentary particles [5, 6]. Due to the variety of cell types within a plant, phytoliths are formed in a multitude of shapes and sizes depending on the location of deposition and the age of the plant. Phytoliths have been extracted from many different plant organs, including leaves, stems, inflorescence (flowers), seeds and roots. Phytoliths can take shape of readily recognized cells, for example, hairs of stomata, bilobate or cross forms [7, 8]. To determine their original location within the plant tissue, phytoliths need ideally to be viewed in place within living tissue, or as a phytolith „Skelton‟. Each plant therefore produces an assemblage of phytolith types, which may or may not be taxonomically significant at family, genus or species level [4]. Dramatic developments during last 20 years have seen scientists learning a lot about the production of phytoliths in modern plants. Current knowledge suggests that phytoliths are restricted to the vascular plants, with high production levels and common family-specific forms occurring in the pteridophytes (tree ferns and horsetails), basal angiosperms (magnolias), and monocotyledons (particularly the grasses and sedges), eudicots (daisies, melons) [9, 10, 11]
Distribution In Plant Kingdom
Phytoliths are wide spread in plant kingdom, occurring in all types of plants and all their different organs and structures, roots to wood to inflorescences. There is accumulating evidence that a silicon deposition system developed at an early stage of plant evolution. For example, Jones (1964) [12] described Phytoliths from 60 million-year-old sedimentary rocks, and Stromberg (2002) [13] recovered copious numbers of Phytoliths belonging to a variety of monocotyledons, dicotyledons, conifers and ferns from sediments ranging from late Eocene (about 35 million years ago) to late Miocene (about 7 million years ago) age.
Unicellular organism such as diatoms that evolved long before plants invading land developed mechanisms for the impregnation of solid silica into their structures hundreds of million years ago [14], and primitive land plants such as bryophytes (liverworts, hornworts and mosses) apparently did the same after their ancestors left the oceans [15].
Numerous angiosperms, gymnosperms and Pteridophytes produce large quantity of Phytoliths [16]. Among the Pteridophytes species of Equisetaceae are heavy silicon accumulators [17].
The dicotyledons have traditionally been thought of as plants that absorb relatively small amounts of silica. However, several investigations have indicated that this view is over simplification. Scurfield et al. (1974) [18] found silica deposists in 32 woody dicotyledons and Postek (1981) [19] has shown deposition to occur in the leaves of Magnolia grandiflora. Examples of herbaceous dicotledon families in which silica deposists have been located include members of Canabaceae [20] and Urtrcaceae [21].
Despite the above observations it is probably still true to say that monocotyledons generally take up and deposit more silicon than the dicotyledons. According to Dahlgren and Clifford (1982) [22], silica deposition is most common in certain monocotyledonous families, including the Restionaceae, Arecaceae, Zingiberaceae, Bromeliaceae, Orchidaceae and Cypraceae. It is, however, in the grass family (Poaceae), the most highly evolved family and very successful family ecologically, that the heaviest and most characteristic deposits of silica occur [23, 24].
Distribution In Plant Body
Lanning and Eleuterius (1981) [25] reported that among the vegetative parts of herbaceous plants, leaves accumulate more than twice as much silica as stems and roots of the plants are found to accumulate least amounts of silica. Levels of silica were found to increase from legumes< fruit crops< vegetables< grasses< grain crops [26].
Studies of reproductive organs show that higher phytolith content may occur in fruits and seeds than in leaves of silicon accumulating species in case of herbaceous plants. Examples include Sedges and Grasses etc. [27, 11, 28]. In cereals, the inflorescence bracts enclosing the seeds of wheat, oats, barley, rye, rice and maize, known in botanical terms as glumes, lemmas and palaes, very often have higher phytolith content than the leaves. The cupules of maize cobs are also a considerable source of phytoliths [16].
With relation to the non-reproductive structures of the herbaceous plants, leaves often contribute more than twice as much silica as stems [25, 16]. Stems of species whose leaves do not have a high silicon content correspondingly show little to no production. It has been assumed that most roots and other sub-terranean organs have negligible phytolith content [29]. Sangster and Hodson, (1992) [30] found in some species, rhizomes and tubers, which are underground stems posses a considerable phytolith content (Marantaceae and Heliconiaceae).
Phytolith distribution in trees and shrubs are still few, but on the whole foliage, fruits/seeds, and, not uncommonly, wood and bark may be expected to have lines share of silica. Significant quantities of silica have been isolated from the wood of numerous species of plants [28, 11]. In woody plants, as in herbaceous taxa, production is often restricted to the epidermis of fruit exocarp and mesocarp, where Phytoliths probably function to protect the propagules of plants from their predators. In these species, phytolith production appears to be less common in seeds than in fruits, although many exceptions occur [29]. It has been found that seed and fruit phytolith are often produced by species whose leaves accumulate significant amounts but are almost never present in species showing no or low foliage phytolith production. Silica deposition in grass caryopsis (grain) has been little studied, but recent investigations have indicated its presence in certain tissues of some species. In caryopsis of Setaria italica, silica deposition takes the form of granular electron-opaque layer external to the outer aleuron cell wall [31].
The epicarp hairs present on the mature caryopsis of the 4 cereals, barley, oats, rye and wheat, have been investigated by Bennett and Parry, (1981) [32]. In all four cases silicon was found to be present along the whole length of hairs, but was most concentrated in the extreme tips.
Distribution in Tissue System
Silica may be deposited in cells of any of the three fundamental tissue systems viz., dermal, ground and the vascular [33].
Epidermal Layer
Epidermal cells in grasses fall into three groups: long or fundamental cells, short or specialized cells and bulliform cells [34]. The fundamental cells are long cells with straight, undulating, or deeply furrowed walls. Fundamental (or long) cells make the greatest proportion of epidermis and occur in rows either singly or in combination with differentiated cells viz., trichomes, silica-cork cells, single cells and stomata [35]. In some grasses, there are few differentiated cells associated with the fundamental cells in the area between the vascular bundles (eg., Bromus inermis). In other grass species the fundamental cells alternate with silica-cork cells and stomata (e.g., Agropyron smithii). Over the vascular bundles, fundamental cells always occur in association with one or more types of specialized cells. The specialized cells are silica cells, cork, exodermic elements, and stomata. Bulliform cells are highly vacuolated cells of the epidermis which usually occur in bands between and parallel to the veins.
Metcalfe (1960) [23] also stated that most cells of the epidermis fall into two sizes: long and short cells. In addition to the two groups of cells based on size, the author described several types of specialized cells namely silica-cork-cells (containing silica bodies), stomata and dermal appendages.
Bulliform cells occur in the upper i.e., adaxial surface of the leaf blade and are generally located midway between the vascular bundles. Bulliform cells have thin, smooth walls, and differ from fundamental cells in being shorter in length and larger in diameter. These cells extend lengthwise forming a band of cells parallel with the vascular bundles, each band varies from a few to many cells. They also have a fan-shaped arrangement in many grasses. The distribution of silicified bulliform cells also varies from single cells to groups. Bulliform cells are the principal source of keystone phytoliths used in identification.
Yoshida et al. (1962) [36] found silicified cells in all parts of the rice plant, including the roots. However the epidermal tissue system had greater proportion of infiltrated cells than the other two systems. Treachery elements are sometimes silicified. In some grasses, e.g., Avena sativa, silicified vessel elements and tracheids are quite common but they are infrequent in other grass species.
Kaufman et al. (1983) [37] conducted electron microprobe analysis of the deposition of silica in epidermal cells of Avena and reported that silica was deposited quite rapidly in the silica cells but very slowly in the cork cells. The location of nucleus is marked by bubble-like black spots in the silica cell. Silica-cork cells are prominent in most parts of Agropyron smithii, Elymus virginicus, Hordeum vulgare, Secale cereale, and Triticum vulgare and in several other species of the tribe Hordeae. Many other grass species also have silica-cork- cells in pairs but they are not as prominent as in the species named above. The author also reported that pairs of silica-cork cells are found in the epidermis of most grass species. They are present in rows over and between the veins often in conjunction with fundamental cells. Leaves of young plants and the basal leaves of flowering culms do not have silica-cork cells. The author also reported that short cells that are not paired with cork cells are found over and between the vascular bundles. However, single cells do not occur as frequently as in the silica-cork combination. Not all of the single cells are silicified but the silica cells of the silica-cork pairs are invariably silicified.
Bonnett (1972) [33] reported that silica is deposited as a layer beneath the cuticle in the epidermis of the rice leaf, leaf sheath and glumes and that silica deposition reduces susceptibility of rice to fungal infections. Some grasses (Aegilops spp., Agropyron spp., Phleum pretense and Triticum vulgare) have short cells that have a thick, sinuous cell wall with simple pits. When the lumen is completely filled, the silica penetrates the pits. Such short cells appear to be covered with prickles which result from silica deposited in the pits. Silica containing cells appear to be completely filled with silica except for one or more bubbles which indicate the location of the nucleus and larger cytoplasmic parts. Some silica bodies like those of Avena sativa have many bubbles. On the other hand, silica bodies of most Festucoideae have a limited number of bubbles. Dumbbell-shaped silica bodies like those of Arisida intermedia may have a bubble in each of the spheres or the bubbles may be grouped in the bar. In saddle-shaped silica bodies, the bubble is near the middle.
Ernst et al. (1995) [7] found that phytoliths occur commonly in the epidermis or outermost covering of seeds and fruits in numerous arborescent and herbaceous plant species.
Trichomes
Trichome is a common term used to designate all unicellular or multicellular appendages. Metcalfe (1960) [23] classified hairs on grasses into three major groups; macro-hairs, micro-hairs and prickle hairs. Prickle-hairs may be hooked or straight. These trichomes point towards the apex of the plant part on which they are found. Hooked trichomes are more common on the margins of the leaf blade, empty glumes, lemmas and the axis of the inflorescence of many grasses. These trichomes are thick-walled and may have silica deposits in their lumen.
Micro-hairs are present on the epidermis of both the leaf surfaces, the abaxial surface of the leaf sheath, empty glumes, and lemmas. They are bicellular structures comprising of a basal cell and an apical cell. They are found between the veins but not over them. The basal cell of the micro-hair lies in the epidermis. The microhairs bend to a right angle to the leaf surface and point towards the apex. The basal cell has a thick wall whereas the apical cell is thin walled. The basal cell may become silicified and is identified as a pipe-shaped particle among the small particles of plant opal. The apical cell is seldom silicified [33].
Mesophyll
Mesophyll is the tissue located between the epidermal layers of the leaf and comprise of thin-walled, lobed cells, containing the chloroplasts [35]. The lobes in the interior of the mesophyll make contact with the lobes of adjacent cells in every plane. Large spaces between lobes give mesophyll a spongy appearance.
The silicification of mesophyll occurs beneath epidermal cells. Variable amount of silicification occurs from the upper cell walls in contact with the epidermis to one or two layers of cells of the mesophyll. Silicified mesophyll cells are usually found in groups either alone or attached to the silicified epidermal cells. Groups of silicified mesophyll cells are found in plant opal 20-50 microns in size. In the plant opal ranging from 5 to 20 microns in size individual cells and fragments are seen.
Silicon In Earth
Silicon is the second most abundant element of the Earth‟s crust after oxygen [38]. Its content in the soils vary greatly and ranges from less than 1% to 45% by dry weight [39]. In nature, silicon does not occur as an elemental form but it is a compound of many minerals which form rocks. Although it is 146 times more abundant than carbon on the earth‟s crust it rarely appears in biological materials. It shares many properties of carbon which form the back bone of the most organic molecules but seldom forms an integral component of any biomolecules as the Si-Si bonds are considerably weaker than C-C bonds and many Si-H bonds are relatively unstable and react readily with oxygen. The larger size of silicon atom compared to carbon also render it unsuitable as a building block despite the fact that it can also form bonds with 4 other atoms creating a three dimensional network similar to carbon [40]. Silicon occurs mainly in the form of silicon dioxide (silica) and silicates that contains silicon (Si), oxygen and metals [41, 42]. Soluble silica, the raw material of silica body formation, is released into the soil by weathering of silicate minerals such and quartz and feldspar [29]. For example, orthoclase feldspar, a mineral present in the soil, is hydrolysed by the hydrogen and hydroxal ions of the water into the mineral kaolinite, resulting in the release of potassium ions and monosilicic acid (Si (OH)4) into solution. Monosilicic acid is soluble in water giving a concentration of 2mM at 250C, higher concentrations implying that polymeric forms of silica are present [43]. These latter two compounds are taken up by the plant roots and transported throughout the plant in xylem sap.
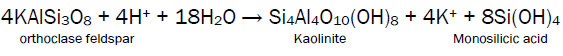
Uptake and Deposition of Silica
Silicon is taken up by the roots in the form of silicic acid (Si (OH)4), an uncharged monomeric molecule, when the solution pH is below 9 [44]. The process starts when soluble silica is absorbed by the plant roots along with other elements occurring in the ground water and carried upwards to the aerial organs in the transpiration stream via the water conducting tissue, the xylem. It is presumed that root hairs assist in the uptake of nutrients and water from the soil [45]. But Ma et al. (2001) [46] found that lateral roots but not the root hairs are responsible for Si uptake in rice. In a study they investigated the role of root hairs and lateral roots in Si uptake using two rice mutants, one defective in the formation of root hairs (RH2) and another in that of lateral roots (RM109) and the wild type. Their results clearly show that lateral roots contribute to silica uptake in rice while as root hairs do not.
Through mechanisms that are incompletely understood but that increasingly appear to be under a considerable genetic and metabolic control [47, 48], some of the silica is eventually laid down in the growing plant as solid, SiO2 in-fillings of cell walls, cell interiors (lumina), and intercellular spaces. Most of the soluble silica initially absorbed from the ground water is transported to aerial structures, where it may result in the heavy impregnation of both vegetative and reproductive structures. Certain plant taxa also heavily silicify their underground organs [29].
There are two major ways by which plants are thought to absorb soluble silica, and although there has been debate concerning which is most important, it is now clear that both are critical to the transfer of monosilicic acid from ground water into roots and then to aerial organs of the plants. The two mechanisms are:
Active Transport
The active transport of monosilicic acid by metabolic processes is in strict control of plant. In this uptake, plant expends energy metabolically during silica absorption. When a plant expends energy in this manner, it is choosing to allocate a portion to a finite set of resources to use silica in some way, and, in itself, it indicates a designated functions for the silica so absorbed, either in its soluble or in its solid state [16]. Active uptake is dominant in rice, sugar cane and wheat [49, 50]. Root exudates that prevent silica polymerization may aid in active uptake, their by allowing more transmembrane movement. The compound poly-2-viny pyridine-1-oxide may act in this manner, which explains its effect of increasing the amount of leaf silicification in rice [51]. Okuda and Takahashi (1964) [52] first showed that silicic acid appeared to be entering the xylem sap of rice shoots against a concentration gradient, a feature pointing to active transport. Okuda and Takahashi (1962) [53] documented that in case of rice silicon accumulation is an active process which is seriously inhibited by respiratory inhibitors like, sodium cyanide (NaCN) and metabolic transporters, providing a clue for the involvement of active transport of silica in plants. The plants with an active Si uptake uptake system and transport are characterized by a much higher intake of Si than of water, resulting in Si depletion in nutrient solutions [46, 54].
Van der Worm (1980) [55] demonstrated active uptake in sugarcane and rice, and subsequent studies by Jarvis (1987) [56] and Ernst et al. (1995) [7] confirmed the importance of active uptake in grasses and sedges, where passive processes were also often at work in different areas of the plants.
Passive Transport
The passive, non-selective flow of monosilicic acid along with other elements from groundwater is through transpiration stream. In this pathway the plant expends no energy metabolically during silica absorption [29]. Plants with a passive mode of uptake take up Si at a rate that is similar to the uptake rate of water; thus, no significant changes in the concentration of Si in the uptake solution is observed [53, 46].
The evidence for passive silica uptake is considerable. In a classical laboratory experiment, Jones and Handreck (1965) [49] showed that they could closely predict solid silica content, expressed as a percentage of the total dry weight of the plant, by knowing simply the concentration of silicic acid in soil solution and the amount of water transpired. Such a close relationship between movement of water and amount of silica that a plant eventually solidifies is expected to be under a passive control. On an average, plants absorb from 50 to 200 kg of Si ha-1. Such values of silicon absorbed cannot be fully explained by passive absorption (such as diffusion or mass flow) because the upper 20 cm soil layer contains only an average of 0.1 to 1.6 kg Si ha-1 as monosilicic acid [57].
Despite the much work conducted over the last two decades, mechanism for silica uptake and transport in plants still remains poorly understood, Because an absence or a very low concentration of phytoliths has been found also to be the characteristic of many plants, it stands to reason that they probably have some mechanism for either the rejection and entry of silicic acid at the root surface or preventing its passage from the roots to the aerial organs. Parry and Winslow (1977) [58] experimentally studied such kinds of mechanisms in peas (Pisum sativum), which do not produce many phytoliths. Root removal in pea seedlings resulted in the high concentration of silicon but not individual, solid deposits of it in leaves and tendrils, whereas plants grown in silica solution with roots still intact showed no such accumulation. This finding indicates that a mechanism of silica rejection occurs in the roots and is probably located near the outer surface of roots since no silicon was detected within the roots of intact plants.
After uptake, dissolved monosilicic acid moves across the cortex of the root until it reaches the endodermis. The endodermis has long been considered to control water and solute movement into the vascular tissues [59]. The casparian bands block apoplastic flow in young root tissues, and only flow through symplast is possible. The secondary stage of endodermal development involves the formation of suberin lamellae around the inside of the cell. It has been recently suggested that this layer is permeable to water [60]. If this is so then apoplastic flow occurs within this layer, inside the casparian bands in older roots. Nevertheless, the endodermis undoubtedly constitutes the major resistance to monosilicic acid flow through the grass roots, and it is here that many of the root silica deposits are found [61].
Frey-Wyssling (1930) [62] suggested that the silica accumulation at the aerial plant surfaces is due to transpiration. Transpiration has been implicated in both the conduction and content of silica in aerial portions of gramineous and other crop plants. The relevant earlier studies have been reviewed by Jones and Handreck (1967) [63]. Developing silica cells in the leaf sheath of wheat (Triticum aestivum) have an apparently normal cuticle but differ from surrounding cells in having smaller nucleoli and thinner outer cellulose walls [64]. Thin outer cellulose walls may result in higher rate of transpiration, facilitating an influx of silica as monosilicic acid [63]. Sangester and Parry (1971) [65] and Raven (1983) [66] advocated transpiration or water loss as a major factor in silica polymerization. In some species, greater amounts of silica are deposited in those regions of the plant were water loss is maximum. However, this is not always the case, since silica is often deposited in tissues that restrict water loss, such as sclerenchyma. Indeed the association between the development of silica bodies and sclerenchyma requires further exploration, since the two are often associated; for example, in orchids [67]. The objection to these hypotheses of passive silica deposition is the difficulty of explaining the pattern of typical silica deposition. However, this could bring about as an indirect result of changes in the structure or biochemistry of potential silica-cells. Prat (1948) [68] suggested that developing silica-cells undergo premature senescence, which is accompanied by a drop in their pH. Such a change could initiate silica precipitation, as silica sols are unstable with respect to changes in pH level, being particularly susceptible to the gelation at pH 5.5. Alternatively, if the structure of the external wall or the cuticle of the cells allowed more rapid cuticular transpiration to occur, silica solutions would concentrate in these cells, resulting in the gel and opal formation.
It is believed that silica accumulation is controlled by plants to increase the mechanical stability of their tissues and to provide protection against microorganisms and herbivores [63, 69]. Thus there are two different hypotheses to explain silica accumulation in plants. If silica accumulation is as a result of water consumption, plants could be expected to accumulate silica not only during growth but also after maturation. If on the other hand, silica plays an active role in the protection of plants, it could be expected that accumulation occurs during plant tissue differentiation and cease when plants are mature.
Commoner and Zucker (1953) [70] proposed that the appropriate enzyme system for deposition might be synthesized or segregated in some cells only. However, till date no enzyme active in silica deposition is known. Molecules which are active in other aspects of silica metabolism in grasses, e.g. in silica uptake [71], may participate in deposition process.
Silica accumulation is characteristic of some plant families, whereas others produce little or no silica. Species with little silica control the amount of silicic acid that enters the root or passes from the root to aerial tissues of the plant [29]. Jones and Hendreck (1969) [72] proposed a hypothetical barrier in root epidermal cells of clover (Trifolium incarnatum) that restricted the flow of silicic acid into the transpirational stream. Experiments conducted by Parry and Winslow (1977) [58] on pea seedlings (Pisum sativum) showed that there is some mechanism at the root surface which disallows the passage of monosilicic acid into the root. In other species, such as Vicia fabia and Ricinus communis, a layer of fatty substance on the root-hair surface may form the barrier [58].
There are substantial differences in the silicon concentration in plant kingdom. The range of its concentration is 0.1-10% Si on a dry matter basis [73, 74]. Plant species older in evolutionary sense (diatoms, cyanosis, horse tails, and ferns) contain more Si than plants that emerged later. Among higher plants, species from gramineae and cyperaceae families accumulate Si in large amounts and are considered as Si accumulators (higher than 1% silicon on dry weigh). Rice and other wetland grasses are an example of Si accumulators. Most dicotyledonous plants contain less than 1% of Si on dry matter (non-acumulators). A third distinguishable group of plants has intermediate plants, Jones and Handreck (1967) [63] listed dryland grasses such as rye and oats. However, recent studies indicate that a high silicon concentration is not a general feature of monocotyledons species [75]. Most Si is deposited in cell walls of roots, leaves, stems and hulls, where it may form a thin layer consisting of silica gel (SiO2.nH2O). Investigations conducted by Ma et al. (2003) [76] on grains of 401 barley varieties showed that the variation in Si concentration in grains is controlled genetically. More than 805 0f the total Si concentration in grains is controlled genetically. More than 80% of total Si was localized in the hull and its amount ranged between 15.343 and 27.089 mg Kg-1 in tested varieties.
Grasses and bamboos are known to have large deposits of silica in the tissues of leaf blades and inflorescence bracts. It is reported that the silica content in mature leaves of Phyllostachys pubescens increases rapidly during the first early growing season, levels off during the first autumn, and then increases again during the following early spring [77], whereas it never increases in P. bambusoids [78]. However, it is unclear whether the increase in the second growing season is the result of leaf ageing or to reactivation, because the life span of leaves in both the species is only one year. In contrast, leaves of Sasa veitchii have a life span of approximately 2 years and continuously accumulate silica throughout their life, not only during the developmental process but also after maturation. This study clarifies the season- dependant changes in the silica content, and shows that the accumulation pattern (rapid in spring and summer and slow in winter) is repeated during the 2 years of life span. These facts support the first hypothesis that silica deposition is a result of water uptake by plants [79].
Silica deposition at the tissue level was investigated in young, mature leaves of Pleioblastus chino (about 1-2 months after leaf expansion), and was found to be densest in the epidermis and least in the mesophyll and vascular tissues [80]. If silica deposition is as a result of water uptake by the plants, silica deposits would be expected to be denser in the mesophyll, where a substantial amount of water transpires directly, than in the epidermis. However, mesophyll cells do not accumulate much silica perhaps because they are prevented from doing so as they are actively involved in photosynthesis. Consequently, these results support the second hypothesis that the silica deposition in tissue systems is positively controlled by the plant.
Chemical and Physical Characteristics of Phytoliths
Phytoliths are often referred to as “plant crystals” when, in fact, siliceous secretions are composed mainly of amorphous (noncrystalline) silicon dioxide (SiO2) with varying amounts of water, usually ranging from 4% to 9%. Early researchers established that phytoliths contain small amounts of Al, Fe, Mn, Mg, P, Cu, N, and organic C, ranging from <1% to about 5% of total phytolith weight [81,82]. These elements are present in the cytoplasm of living cells and then retained when cells become impregnated with solid silica, becoming encased within the phytolith [81, 83, 84]. The carbon in phytoliths is a suitable material for radiocarbon dating using conventional radiometric methods or accelerator mass spectrometry (AMS) [85, 86, 82, 87]. Stable carbon, oxygen, and hydrogen isotope ratios can also be directly determined from modern and ancient phytoliths, and carbon stable isotopes have so far been shown to be especially useful in deciphering past vegetation and climate [84, 88]. Biogenic silica of plant origin is optically isotropic, ranges in refractive index from 1.41 to 1.47, has a specific gravity from 1.5 to 2.3, and ranges in color under transmitted light from colorless or light brown to opaque [89]. Many phytoliths are notably transparent, and it is often possible to look through them and ascertain three-dimensional shape and other features without turning them in the mounting medium. All darker forms of phytoliths, which may be common in some soils and sediments, were originally thought to be caused by more opaque forms of organic material occurring within phytoliths [89]. When found in soils and sediments, it is likely that many of these have actually resulted from plant burning, which leaves physical evidence on phytoliths in way of a partial or total charring of the surface [28, 90]. Phytoliths may thus serve as an index of the occurrence and intensity of prehistoric vegetation firing. Phytoliths containing visible carbon inside them from trapped cellular contents do occur and can usually be identified as such by the presence of granules or dark spherical cavities, possibly sometimes representing the nucleus of the cell [91, 89]. Phytoliths with no obvious signs of carbon also contain cellular cytoplasmic matter. Burnt and organic darkened phytoliths have a lower specific gravity than lighter colored forms [89].
Physiological Role of Silica in Plants
Arnon and Stout (1939) [92] observed that inspite of the high silicon accumulation in plants (its amount may equal concentration of macronutrient), until now it has not been considered as an essential element for higher plants according to the criteria of essentiality. Silicon is recognized as a beneficial element for plants growing under biotic and abiotic stresses, for example heavy metal, drought, salinity and pathogens. Ma (2004) [93] reported the beneficial effects of silicon have been observed on growth, development, and yield and disease resistance in wide variety of plant species. Si fertilizers are routinely applied to several crops including rice and sugarcane to enhance high and sustainable crop yields.
Growth and Development
Silicon is an indispensable element for the normal growth and development of some plant species. Examples include horsetails or scouring rushes (Equistem), rice (Oryza sativa) and beets (Beta vulgaris). Negative effects of silica poor growth medium can be dramatic. For example, shoots of equistem, a heavy silica accumulator, collapsed when grown in a silca-free medium [19].
Silicon has been found to provide structural rigidity to plant parts and hence increasing light interception and energy manufacture, for example, rice leaf blades are more erect silica slag‟s are applied, allowing more light to reach the lower leaves and substantially increasing the photosynthetic activity there [94]. According to „window‟ hypothesis, presence of epidermal silica bodies facilitates the transmission of light through the epidermis to the photosynthetic mesophyll or to stem cortical tissue, consequently increasing photosynthesis and plant growth [95].
Silica deposited in epidermal cells may act to reduce the rate of transpiration in leaves thereby improving water use efficiency [96]. Silica may help to maintain rigidity in stems and linear leaves, although leaf stiffness may also be related to the degree of lignifications. Silica has been shown to improve the lodging resistance in wheat. In experiments with both solution cultured and soil-grown plants, a recurring observation has been that plants supplied amply with Si resist lodging (drooping), leaning, or even becoming prostate. The mechanical strength of plants enabling them to achieve and maintain an erect habitat conductive to light interception resides in the cell wall. Reviews on the role of silicon in plants therefore stress the association of Si with cell walls and discuss the increased rigidity of cell walls of plants grown under ample available Si in terms of that association.
The incorporation of silica into cell walls has at least two energetically positive effects. First, the role of silica is analogous to that of lignin in that it is a compression-resistant structural component of cell walls. Raven (1983) [66] has calculated that on a unit weight basis, the energetic cost of incorporating silica is only 3.7% that of incorporating lignin. For incorporation of silica compared with that of cell-wall carbohydrate, the corresponding value is 6.7%. Silica is thus an energetically inexpensive structural component of cell walls. Second, the erect habit and the disposition of the leaves of plants amply supplied with Si favour light interception and, hence, photosynthesis. Thus in the experiment with cucumber plants grown in a recirculating nutrient solution already referred to [97], the authors found that the responses of the leaves of the high-Si plants resembled those elicited by high levels of solar radiation. Although no systematic comparisons have been made, it is likely that many of the positive effects of Si on plant growth that have been recorded were due to increased total energy capture. Optimization of silicon nutrition results in increased mass and volume of roots, giving increased total and adsorbing surfaces. As a result of application of silicon fertilizer, the dry weight of barley increased by 21% and 54% over 20 and 30 days of growth, respectively, relative to plants receiving no supplemental silicon. Silicon fertilizer increases root respiration. Silica plays an important role in hull formation in rice, and, in turn, seems to influence grain quality. The hulls of poor-quality, milky-white grains (kernals) are generally low in silicon content, which is directly proportional to the silicon concentration in the rice straw [98].
Sangster et al. (2001) [99] reported that physiological functions involve the interaction of silica with other processes taking place in a plant or its growing environment. A good physiological reason that why plants might want to make phytoliths has recently been brought to the force. Silicon dioxide may ameliorate the toxic effects of aluminium and other heavy metals, such as manganese on plant growth. A number of experimental studies indicate that the addition of silicon in growth medium mitigates the damaging effects of aluminium and manganese on plant growth. A co-deposition of solid silica and aluminium in and around plant cells has been demonstrated for a wide variety of species and plant organs; hence, the mechanism for detoxification may be sequestration of aluminium by silica. Moreover, it now clear those plants of all types accrue a number of benefits to growth and reproduction that appear to be directly related to the presence of silica in either a soluble or a solid state. All the above debate suggests structural and physiological functions of phytoliths.
Biotic and Abiotic Stress
Silica is able to protect the plants from multiple biotic and abiotic stresses. Number of studies has shown that silica is effective in controlling diseases caused by both fungi and bacteria in different plant species. Djamin and Pathak (1967) [100] tested that those with high silica content showed greater resistance to Asiatic stem borer (Chilo suppressalis) than others, probably because the silica interfered with the boring and feeding of the larvae; selecting rice varieties with a high silica content was more economical than applying silicate to the soil. Hanifa et al. (1974) [101] studied the role of silica in the resistance of the rice to the leaf roller (Cnaphalocrocis medinalis), and Moore (1984) [102] examined the relationship of silica to stem-borer infection by OscinellaI species in Lolium. Most of the plant silicon occurs in the epidermis, which might dislodge young larvae before they can establish in the stem. Various studies have demonstrated that silicon increases the hardness of plant tissue, which negatively impacts insect larval boring and feeding ability.
Application of silicon to crop plants not only increased the yield but also showed a marked reduction in the incidence of grain discolouration. The discolouration is the result of infection of husks by several fungi. In a subsequent investigation a number of rice disease organisms were identified [103]. Application of silicon to the soil reduced the severity of all diseases identified; different rise genotypes responded to various degrees. Recently, a parallel mechanism as that seen in the resistance of plants to diseases via activation of the plant‟s own defense mechanisms by soluble silicon has been observed for insect pests. Sieburth et al. (1990) [104] reported such a mode of action against insects such as the noctuid (Trichoplusiani), the coccinellid (Epilachna varivestis), the aphid (Acyrthosiphon pisum), and the cockroach (Periplaneta Americana). Si treated plants of cucumbers demonstrated enhanced activity of chitinases, peroxidases, polyphenol oxidases and flavinoid phytoalexins, all of which may protect against fungal pathogens. Rodriguez et al. (2003) [105] studied wheat and rice blast, respectively, and indicated that these species were also capable of inducing similar biologically active defense abets, including increased production of glycosylated phenolics and antimicrobial products such as diterpenoid phytoalexins in the presence of silica.
Kauss et al. (2003) [106] experimenting on the cucumber leaves following fungal infection, showed that further resistance to fungal infection is acquired by the expression of a proline rich protein together with the presence of silica at the site of attempted penetration. The c-terminus of this protein contained a high density of lysine and arginine residues proposed to catalyse the localised deposition of silica at the site of vulnerability. Similarly, Keeping and Meyer (2006) [107] reported the resistance of sugarcane to E. saccharina.
In addition, metal toxicity, salinity, drought and temperature stresses can be alleviated by silicon application and the means by which silicon exerts these protective effects is still under investigation.
Epstein (1994) [73] reported that aluminium toxicity is found to decrease on increasing Si in the nutrient supply to cotton, maize, soybean and barley. Perry and Keeling-Tucker (1998) [108] reported that aluminium oxides, either added or already present in the soil, also reduce the availability of Si for plant uptake; a situation occurring naturally in heavily weathered and/or acidified soils in Australia. A deficiency of Si causes an increased uptake of Manganese and Iron in rice, barley, rye and ryegrass causing toxicities. Application of Si fertilizers relieves this toxicity The proposed mechanism may be increased oxidation of Mn at the root surface if there is sufficient oxygen present, and redistribution of Mn to prevent necrosis.
Neuman and Zur Nieden (2001) [109] studied toxicities of metals like Mn, Cd, Al, and Zn and found that proposed mechanism for the action of silica include the accumulation of Zn as a silicate; increased release of phenolics with strong chelating ability for Al tolerance; reduction of lipid peroxidation and increased enzymatic (e.g. superoxide dismutase; SOD) and non- enzymatic antioxidants (e.g. ascorbate) against Mn toxicity.
Wang et al. (2005) [110] reported that drought tolerance brought about by the application of „Si‟ may result from decreased transpiration and presence of silicified structures in plants. It is worth noticing that the beneficial functions of silicon do not reveal itself under optimal circumstances but mainly under stress conditions. The mechanism responsible for plant resistance to water stress and a possible role of Si in these processes may be considered at different levels (molecular, cellular, whole-plant). Essential features of plants response to water stress are following: i) maintenance of homeostasis, including ionic balance and osmotic adjustment, ii) counteraction to damages and their prompt repair, e.g. elimination of reactive oxygen species (ROS) and prevention of oxidative stress, iii) detoxification of excess salts under salinity, iv) regulation and recovery of growth [111].
Fleck et al. (2011) [112] reported that Si nutrition of rice plants reduced the oxidation power of roots and enhanced the development of casparian bands in the exodermis and endodermis, as well as lignin depositions in the sclerenchyma. These changes are probably the reason for the reduced radial oxygen loss and might be useful for the plants to grow in anaerobic soils and cope with unfavourable conditions. Increased suberization and lignification was accompanied by silicic acid triggered transcription of genes related to lignin and suberin metabolism. In addition, a high impact of silicic acid supply on transcript level of a LRR-RLK gene could be observed, highlighting the possibility that this regulating protein plays a central role either in perceiving a Si signal of an up-to now unknown nature or in promoting suberin and lignin synthesis or in both.
Taxonomy
In the recent advancements in identification of plant species, phytoliths have found an increasing role in the identification of different taxa at different levels of taxonomic hierarchy. Grob (1896) [113] was the first man to demonstrate the potential of phytoliths in plant systematics. Prat (1932) [114] and Metcalfe (1960) [23] also investigated the potential of phytoliths as taxonomic features of grass epidermis. Epidermal phytolith types are utilized for identification of fossil and modern grasses at the sub-family and tribe levels. Thomasson (1978, 1980) [115, 116] used silica cell patterns in epidermal leaf fragments, as well as other epidermal characteristics to classify Miocene grasses. Different subfamilies of grasses (Poaceae) have been characterized on the basis of some diagnostic phytolith types. For example bilobate and saddle shaped phytoliths act as diagnostic markers for panicoideae and chloridoideae subfamilies respectively [16, 117] Although individual phytoliths often cannot reliably help in classification of taxa, an adequately large sample of phytoliths from a given taxa can be distinguished from closely related taxa through the use of classification keys based on the mean morphometries of the phytolith sample or the use of the phytolith morphometries in discriminant functions [118]. Piperno (1988) [29] illustrated that certain kinds of silica bodies may be found in all of the sub-families of Poaceae and they can be used for discrimination below the family level only with caution. However, other short-cell phytoliths disarticulated from plant tissues are valid indicators of individual sub-families, tribes, and genera of grasses, and these do provide valuable information on grass taxonomy and phylogeny. Stromberg (2005) [119] suggested that greater taxonomical identification of grass phytoliths could lead to discriminating the shade-loving basal grasses from the light-loving crown grasses of phylogeny tree, and therefore improve the environmental interpretation of phytoliths. Chauhan et al. (2011) [120] found that a number of phytolith morphotypes are present in the leaves and stem, which are very characteristic and are useful for the taxonomic identification of the plant. The most common type is the bilobate phytolith, other forms include the trapezoids, prickle micro hairs, long micro hairs, epidermal long cells, stomata, bulliform, parallelepipedal cells and knobbed spine phytoliths.