ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
S. Vinay Kumar1, K. Venkateswara Rao2, CH. Shilpa Chakra3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The PZT composites are almost used with a dopant, a modifier or other chemical constituent or constituents like La, Mn, etc. to improve and optimize their basic properties for specific applications. Lead lanthanum zirconium titanium oxide (PLZT) was initially developed by Haertling et.al., as a promising novel electro-optic material. Since that time, studies have focused on various applications, ranging from capacitors, transducers, actuators and electrooptic switches to pyro-electric detectors etc, as it has high dielectric constant, high piezo-electric, ferro-electric, pyroelectric properties. The Lead Lanthanum Zirconium Titanium Oxide (PLZT) composite [(Pb0.92La0.08)(Zr0.65Ti0.35)O3] is synthesized by simple Sol-Gel process using constituent nitrates and a solvent. The resulting powder is characterized using XRD, Particle Size Analyzer, SEM, TG-DTA, UV- Visible spectroscopy and HR-TEM. The structural parameters such as the lattice constants (a, c), X-ray density ( ), Porosity %, average crystallite size (D) have been determined using X-ray diffraction (XRD). Particle size is determined by Particle size Analyzer (PSA), Average grain size and surface morphological studies were carried out using Scanning Electron Microscopy (SEM) and d- spacing value of the material is determined using HR-TEM techniques.
Keywords |
| PLZT, Sol-gel, High Di-electricity, Peroviskite, Stoichiometric Ratio, Polyvinylpyrrolidone |
INTRODUCTION |
| Lead zirconium titanium oxide (PZT) was developed by Haertling, as a promising novel electro-optic material it is the most important member in the lead based peroviskite ferroelectric family [1]. ABO3 group materials are mainly used as an engineering material in electro-ceramic industry [2]. The PLZT unit cell consist of a corner-linked network of oxygen rhombohedral with Zr+4 and Ti +4 ions occupying B site within the rhombohedral cage and Pb+2 and La+3 ions occupies A site created by linked rhombohedral. Here yellow color atoms represent A group elements, black color atoms represent B group elements and white atoms represent oxygen (O) elements. As a result of the different values between Pb+2 and La+3 some of the A site and B site are vacant to maintain electrical neutrality in the structure. The nitrates of Pb, La, Zr, and oxide of Ti are taken in required amounts of stoichiometric ratio for the synthesis of [(Pb La)(Zr Ti)O3] composite. There have been several reports on PZT modified with some lanthanide ions. Haertling, Land reported that Pb+2 ions could be substituted by the La+3 ions of its comparable ionic size. The PZT composite are almost used with a dopant, a modifier or other chemical constituent or constituents like La, Mn, Ba etc. to improve and optimize their basic properties for specific applications. Examples of these additives include off-valent donors, such as Nb5+ replacing Zr4+or La3+ replacing Pb+2 [3,4]. The donors are usually compensated by A-site vacancies. These additives (and vacancies) enhance domain reorientation ceramics produced with these additives are characterized by square hysteresis loops, low coercive field, high remnant polarization, high dielectric constants, maximum coupling factors, higher dielectric loss, high mechanical compliance and reduced aging [5]. Dopants are usually added in concentrations of ≤ 3%. Modifiers are substituted into the original PZT composition as solid solution constituents in concentrations of ≥5%.One system that embraces all compositional aspects of the dielectric, piezo-electric, pyro electric, ferroelectric and electro-optic ceramics is the PLZT system[6,7]. The formation of PLZT composite depends on physiochemical characteristics [8].A number of preparation techniques for PLZT have been reported, such as Ball- Milling, Chemical method, Mechano chemical process, Hydrothermal reaction, High Energy Ball-Milling. |
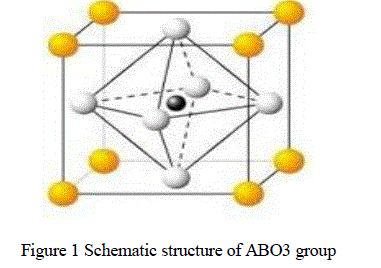 |
| Among these methods, Sol-Gel is well accepted to be a simple and promising method in the preparation of PLZT composite. This method is a wet chemical technique for the fabrication of ceramic materials. In this process, the sol (or solution) evolves gradually towards the formation of a gel-like network containing both a liquid phase and a solid phase. Typical precursors are metal alkoxides and metal chlorides, which undergo hydrolysis and poly-condensation reactions to form a colloid. The basic structure or morphology of the solid phase can range anywhere from discrete colloidal particles to continuous chain-like polymer networks. The advantages of Sol-Gel method includes (a) Higher deposition rates, (b) Good stoichiometric control, (c) Lower initial facility costs and lower processing temperature. |
EXPERMENTAL PROCEDURE |
| Lead Lanthanum zirconium titanium oxide composite was prepared using Sol-Gel Process. The Starting precursors were Lead Nitrate [Pb(NO3)2], Lanthanum Nitrate [La(NO3)3.6H2O], Zirconyl Nitrate [ZrO(NO3)2.2H2O] and Titanium Isopropoxide [C12H28O4Ti][9]. The powder composition [Pb0.92La0.08(Zr0.65 Ti0.35)O3] was prepared by dissolving Lead, Lanthanum and Zirconyl nitrates in double distilled water in the desired ratio. Titanium Isopropoxide was then added directly to the nitrate solution drop-by-drop while stirring. The Ammonia solution was added to the aqueous nitrate solution to maintain pH-value of the solution 7. Polyvinylpyrrolidone (PVP) was taken in the ratio of 1:2 to the solvent to form a gel. The solution was heated at 80oC with constant stirring till zero-gel is formed. The dried cake was crushed thoroughly in a mortar and was calcined at 650oC for 2 hours in muffle furnace. The preparation of PLZT composite is diagrammatical represented in the Fig.1. The powder was crushed and X-ray diffraction patterns of samples were recorded using X-ray diffracto-meter (model: Bruker d8 Advance) with Cu-ka (λ=1.5418Å) radiation source. The SEM studies were carried using Scanning Electron Microscope (Model: JEOL JSM-6360A). The average grain size (grain diameter) of the samples was determined from SEM images. The HR-TEM studies were carried out using JEOL (Germany) GmbH - JEOL JEM-2100. |
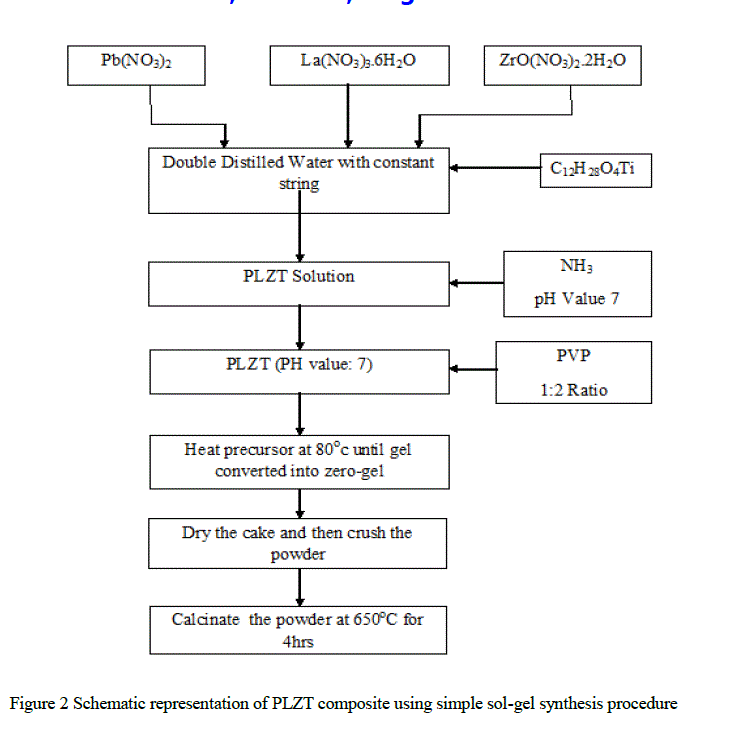 |
RESULTS AND DISCUSSION |
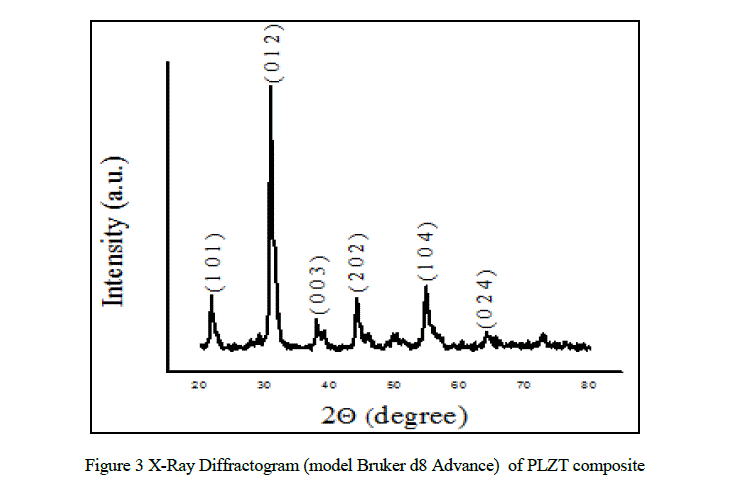
| The X-ray diffraction pattern for PLZT composite is shown in Fig.3. The X-ray diffraction patterns sample clearly indicates formation of crystallite phase. The XRD patterns showed the absence of non-reacted products or intermediate crystalline phases. The positions of all the Bragg lines were used to obtain the inter-planer spacing. The peaks were indexed by comparing the inter-planar distance with the JCPDS data for PLZT (Card No: 53-0785). |
| The lattice parameters were calculated by the relation [10]. |
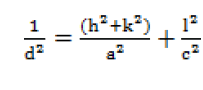 |
| where, d is inter-planer distance, a and c are lattice parameters, (h, k , l) are Millar indices. |
| The X-ray density (ρx) was calculated using the expression [11]. |
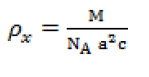 |
 |
 |
 |
| Fig.5. Illustrates the SEM micrographs for PLZT samples. Maximum densification and spherical shape grains are observed in the PLZT sample using SEM analysis. The average grain size was estimated as ~ 0.36 μm. The small grain size observed in PLZT sample leads to small domain spacing and the reduction of distance through which the walls move, resulting in the increase of piezoelectric coefficient. |
 |
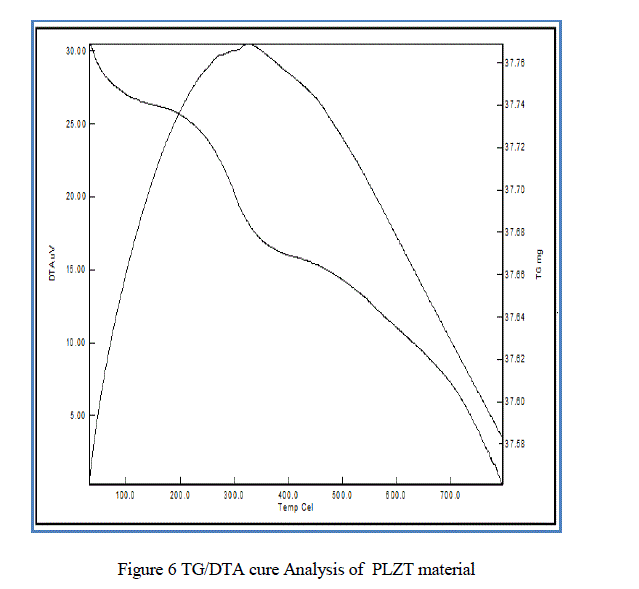
| From the Fig.6. TG/DTA curve we analyzed the weight loss of the material with respect to temperature variation. where the total weight loss of the PLZT composite is of 0.5 mg. |
 |
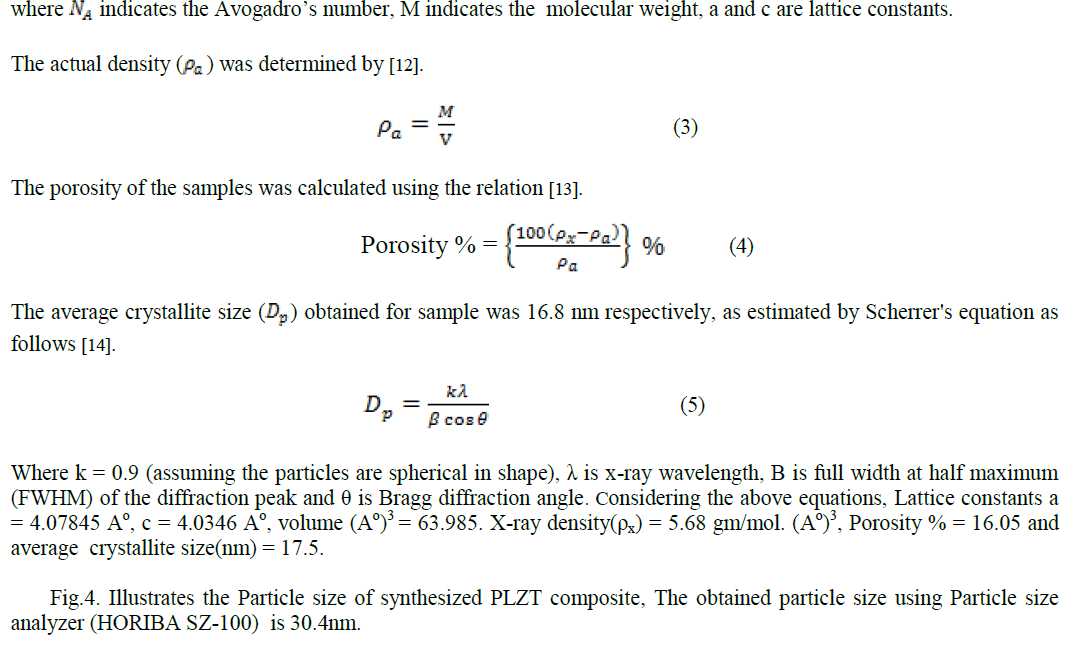
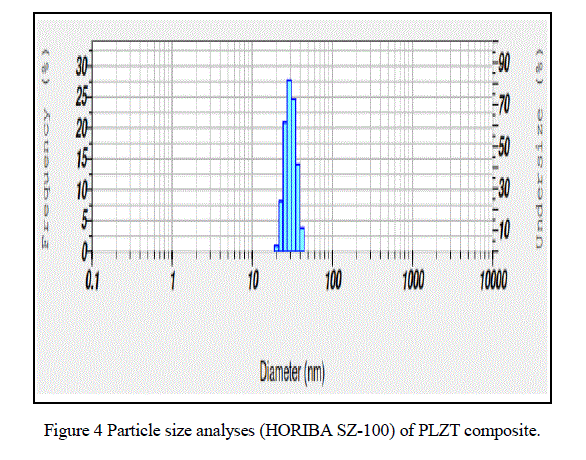
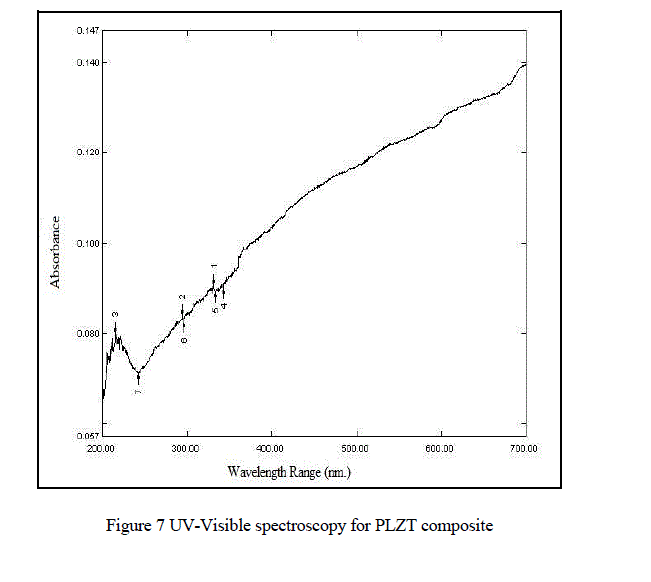
| From Fig.7. UV-Visible spectroscopy (UV-2400PC Series) we observed that the maximum absorption wavelength of PLZT material is 331.50nm . |
| TEM images in Fig.8. Determines the d spacing value between the parallel planes as 0.22nm which was similar to the d spacing value determined from the XRD data. |
 |
CONCLUSIONS |
| In this paper PLZT composite was prepared using simple sol-gel method and the material characteristic were studied. The obtained average crystallite size as 17.5nm, particle size 30.4nm, average grain size ~ 0.36 μm, total weight loss 0.5mg, maximum absorption 331.50nm and d-spacing 0.22nm. |
ACKNOWLEDGMENT |
| This work is supported by the DST Nano- Mission and Centre for Nano Science and Technology, Institute of Science and Technology, Jawaharlal Nehru Technological University, Hyderabad. I would like to thank my guides and friends for their guidance regarding the work carried out by me. |
References |
|