e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, PSGR Krishnammal College for Women, Peelamedu, Coimbatore-641 004, Tamilnadu, India
Received date: 05/04/2014; Revised date: 25/04/2014; Accepted date: 27/04/2014
Visit for more related articles at Research & Reviews: Journal of Chemistry
In the field of nanotechnology, polymer matrix based nano composites have become a prominent area of current research and development. By inserting the nanometric inorganic compounds, the properties of polymers improve and hence this has a lot of applications depending upon the inorganic material present in the polymers. Synergistic improvements in the composite properties were achieved and are superior to those of the individual components. Keeping this view in mind, we have synthesized novel polyester - MMT Clay composite. The polymer has been synthesized by condensation of a synthesized diol with diacid chloride. The synthesized polymers and composites were characterized by infrared spectra, proton magnetic spectra, scanning electron microscope, thermogravimetry and X-ray diffraction analysis. The results indicate that silicate layers are intercalated in the polyester matrix. The polymer composite is found to be crystalline and dense. The thermal stability of the novel hybrid material is higher compared to that of polymer
Nanocomposite, MMT clay, Polymer, 1H NMR, SEM, XRD
The production of clay-polymer nanocomposites is an active area of research which benefits from the high surface area of nanoclay particles to greatly improve the thermo mechanical properties of the polymer matrix, even with low filler contents [1]. Polymers are used as a matrix in these types of composites, and clay minerals act as reinforcement material. By combining these two different structures, new materials can be synthesized that have better physical and chemical properties according to their components [2]. Polymer composites are widely used in electronic and information products, consumer commodities and in the construction industry. Layered silicate/polymer nanocomposites exhibit superior mechanical characteristics, heat resistance and chemical resistance compared to the neat polymer [3].The intercalation of organic species into layered inorganic materials provides a useful and convenient route to prepare organic-inorganic hybrids that combine the properties of both inorganic host and organic guest [4].
Clays have been extensively used in the polymer industry either as a reinforcing agent to improve the physico mechanical properties of the final polymer or as a filler to reduce the amount of polymer used in the shaped structures, i.e. to act as a diluent for the polymer, thereby lowering the high cost of the polymer systems. Clay minerals, and particularly smectites, seem to be suitable fillers for improving the different polymer properties. It is observed that a small amount of well-dispersed clay mineral in the polymer matrix drastically improves its properties [5].
In polymer clay composites, where the sodium cations on the MMT had been replaced with organic ammonium salts. These organic ammonium salts serve as the organic treatment for the clay, making the normally hydrophilic clay hydrophobic. This allowed the polymer to wet the surface of the clay and disperse the clay into the polymer [6]. In recent years considerable efforts have been devoted to the development of methods for the preparation of composite particles consisting of polymer cores covered with shells of different chemical composition. Hence, there is a need for newer and novel polymeric composites for various applications. Herein, we report the synthesis of EBAP - MMT composite and its characterization using XRD, SEM techniques and particle size analysis.
This paper aims to summarize the highlights of our findings from the investigation on synthesis of EBAP - MMT composite and its characterization using XRD, SEM techniques and thermal analysis.
Synthesis of Monomer
The monomer was synthesized by the condensation of 4-hydroxy benzaldehyde with 4,4’- diaminobiphenyl ether in alcohol medium. After the reaction is complete, the formed monomer is filtered, dried and recrystallized from ethanol. The synthesized compound was characterized by the usual spectroscopic and analytical techniques.
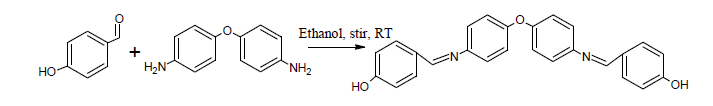
Synthesis of Polymer
The polymer (EBAP) was synthesized by condensation of diacid (Adipic acid) with the diol monomer (EBAPSB). The reaction scheme is shown below.
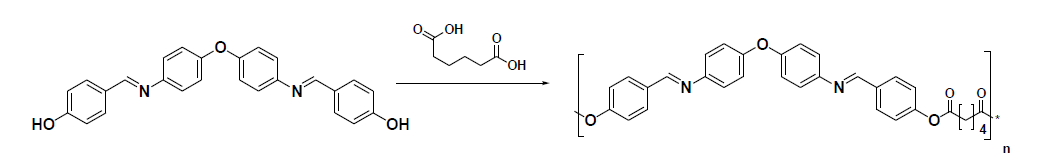
EBAP (Polymer):
Color: Brownish Yellow.
Yield: 83%.
M.p.: 226 °C.
FT-IR (KBr, ν, cm-1): 1680 (OH), 1186 (-C-O-C-).
1H NMR (400 MHz, DMSO-d6, δ, ppm): 9.9 (s, J = 1 Hz, 2H; OH), 1.6 (s, 8H; CH2), 2.35 (s, CH2COO) 6.9 & 7.8 (4d, 8H; Ar H)
Polymer- MMT Composite Preparation
Preparation of composite is carried out in two steps
Modification of MMT Clay
MMT is a layered alumino silicate mineral present in clays and most often used in polymer composite preparation. The surface of MMT is hydrophilic and not suitable for preparation of nanocomposites with most commercial polymers. To ensure good intercalation, the surface of MMT has to be modified. The most common method is a cation exchange with organic ammonium salts, which differ in number, length and structure of long chains. The role of modification of MMT with ammonium salt is to increase the interlayer spacing, reduce interaction between the MMT plates and improve the interaction between clay and polymer [1].
10g Montmorrilonite clay was dispersed in DI water by stirring. The amount of CTAB added was calculated with the cation exchange capacity (CEC) value (110 meq/100 g clay) of MMT, and it was 2% higher than the exact amount by mass. The calculated amount was added to 200 mL of water in the separate container and was dissolved by continuous stirring. After this, homogeneous surfactant, Cetyltrimethyl ammonium bromide solution was added to the dispersed MMT solution. The whole dispersion was heated at 80ᴼC for 4 h. The exchanged clays were filtered and washed with DI water. The modified clay (CEMMT) was dried at 80ᴼ C under vacuum [7].
Synthesis of Polymer MMT Clay Composite
The organo clay is first suspended in the solvent (DMSO). The weight percentage of organo clay used with respect to the polymer is 1:3 (Clay: polymer). Then, the polymer, dissolved in the solvent, was added to the solution and sonicated for about 5 minutes whereby it gets intercalated between the swollen clay layers. The last step consists in removing the solvent by evaporation under vacuum.
Characterization
The synthesized polymer and its composite were characterized by IR, 1H NMR, SEM, TGA and XRD. IR spectra of samples were recorded in the range of 4000 to 400 cm-1 on ATR-IR Affinity1 spectrophotometer. The 1H NMR spectra of the samples were carried out in BrukerAvance III model instrument. The surface morphology of the EBAP and EBAP-MMT samples was investigated using the scanning electron microscopy (Medzer biomedical research microscope). X-ray diffraction studies were carried out for polymer and the composite using XRD 6000 (Shimadzu, Japan). The measurement conditions were CuKα radiation with graphite mono chromator 30 KV voltages and 40 mA current. The thermograms were recorded in dynamic nitrogen atmosphere (flow rate 20 mL/min) with a heating rate of 10 K/min using a Perkin Elmer (TGS-2 model) thermal analyzer.
Results and Discussion
FT-IR Spectra
The FTIR spectra of the polymer, cationic modified MMT and the composite are shown in Fig 1. The band due to phenolic –OH group is absent in the spectra of polymer indicating that it is involved in the polyesterification. The band due to the stretching frequency of –C=N group around 1608 cm-1 is also present in the spectra of polymer. The polymer shows bands at 1680cm-1 due to ester C=O group. In addition to the above bands, the IR bands due to phenyl ring systems in the range between 1520 and 1570cm-1 are almost unaffected in the polymer.
The FTIR spectra of the CEMMT shows peak at 1020cm-1 correspond to Si- O stretching and interlayer water deformation vibrations at 1741cm-1. The band at 3480 cm-1 results from the O-H stretching vibration [8]. The composite shows bands characteristic of the polymers at 1740cm-1(ν C=0),1682 cm-1 (ν C=N) and 1520 -1570 cm-1 due to the aromatic –C=C ring stretch. In addition, the composite shows bands at 1012, and 1210 cm-1 which are characteristic of Si-O-Si stretching [9-14]. This confirms that the MMT layers have been intercalated into the polymer matrix
1H NMR
In the NMR spectra of the polymer (Fig.2) azomethine protons are located at 9.9 ppm. The –OH signal is found at 8.5 ppm as a broad singlet. The most diagnostic are signals of the p-substituted benzene that appear between 6.9 and 7.8 pm.
It shows more intense peaks in the range 5.5 ppm due to CH2 protons indicating the condensation product. In the case of polymer EBAP, four doublets appear in the aromatic system (6.9, 7.2, 7.45, 7.8 ppm) this shows that the ring protons appear as AA’BB’ pattern [15].
XRD
Figure 3 shows the XRD curves of the polymer and composite. It is evident that the composite is crystalline and dense. The polymer shows broad and less intense band in the XRD showing that it is semi crystalline. The XRD pattern of composite shows sharp peaks indicating crystalline structure. The sharp crystalline peak for pure MMT at 2Ө =28º [16] is seen in the composite. It is evident from the XRD studies that there is interaction between the clay and polymer making the matrix stronger. Particle size of the composite (54nm) has been calculated by Scherrer equation using FWHM.
According to Kato etal [17], that the polar groups in the polymers will interact with the polar group in the nano clay and the interaction decreases the d-spacing in the composite producing stronger denser composite. Similar result have been observed by Supri et al [18]
in which they studied the effect of poly acrylic acid on the LDPE-nanoclay composites.
TGA
Thermal behavior of polymer composites is one of the most important factors for processing and applications. Figure 4 shows the thermograms of the polymer, MMT and the composite. From the TGA curves it is evident that the polymer decomposition occurs at 262ºC but the composite shows quite good stability upto 350 ºC.
It has been reported that introduction of clay into polymeric matrices improves thermal stabilities because the clay can hinder the permeability of volatile degradation products out of the materials. The dispersed clay generates a barrier which delays the release of thermal degradation products [11].
In the present study, the effect of MMT on the synthesized polyester has been attributed to insulation and barrier to mass transport against the volatile compounds generated during the decomposition of polymer [19].
SEM
The surface morphology of the nanocomposites is different from that of the starting polymer. No large aggregates and a homogenous distribution of the MMT in the polyester matrix were observed, implying good adhesion between organo-modified filler and matrix [20].
The polyester nanocomposite hybrid material was synthesized & characterized by spectral and thermal studies. The results clearly indicate that silicate layers are intercalated in the polyester matrix. The synthesized polymer composite is found to be crystalline and dense. The thermal stability of the novel hybrid material is higher compared to that of polymer.
The authors thank University Grants Commission (UGC), New Delhi, India for their financial support and STIC, Cochin, Kerala for their technical assistance.