e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Chemistry, St. Teresa’s College, Ernakulam, Kerala, India
2Department of Chemistry, St. Albert’s College, Ernakulam, Kerala, India
Received date: 25/04/2018; Accepted date: 02/05/2018; Published date: 18/05/2018
Visit for more related articles at Research & Reviews: Journal of Chemistry
In the present endeavour PS-HDODA supported Co (II) as a novel heterogeneous catalyst system was developed. FTIR spectroscopy, CHNS analysis, ion exchange capacity measurements and Scanning electron microscopy were the various analytical methods used. The synthesised heterogeneous catalyst particles were of spherical morphology. To exemplify the advantages of this novel support, degradation of methylene blue (MB) dye was conducted using it. The degradation efficiency was found to be efficient and this support can be used for purification of waste water from dye industry.
Cross-linked polymers; PS-HDODA, Sulphonation, Catalysis, Methylene blue
Cross-linked polymers were widely used as catalyst support, as they are inert, non-toxic, have thermal stability and can be recycled as well [1]. The ease of separation of the catalyst from the product systems leads to operational flexibility [2]. More over the amount of metal present on the surface of the catalyst is very small which is of economic significance in the case of expensive metals. Metal complexes anchored on polymers will give an organic polymer inorganic functions [3]. Polymer supported metal complexes show a higher catalytic activity in comparison with homogeneous and unsupported catalyst systems [4,5]. This higher activity of polymer supported catalyst [6,7] in comparison with other supported systems [8,9] is due to the dynamic microenvironment of the supported system [10], in which the supported catalyst is able to perform rotational and translational motion. Cross-linked polymers were more efficient in controlling the activity of metal complexes than linear polymers [11,12] because linear polymeric systems were not able to influence the concentration of reactants on active site as influenced by cross-linked polymeric system. The flexibility of polymer back bone is one of the key factors for better catalytic activity of metal complexes on polymeric support [13-16]. Cross-linked polymer supports showed a remarkable effect on activity [17-19] and selectivity [20-22] of metal complexes, which is barely observed with soluble [23] and inorganic support such as zeolites [24]. Styrene-Divinyl benzene (DVB) copolymer is most commonly employed as support in heterogeneous catalysis. Resins based on polystyrene-DVB (PS-DVB) may be showing low metal ion uptake. This may be due to hydrophobic character and rigid nature of the polymer backbone. The PS cross-linked with 1, 6-hexanediol diacrylate (HDODA) possess optimum hydrophobic hydrophilic balance and is more flexible than PS–DVB system. So PS-HDODA has been found to be more convenient as catalyst support than PS-DVB. The catalytic activity for degradation of Methylene blue (MB) dye was studied using the synthesized catalyst.
Materials
Styrene and HDODA were purchased form Sigma Aldrich. Acetone, methanol, toluene, DMF, DCM, benzoyl peroxide, PVA, sulphuric acid and cobalt chloride were obtained from Merck chemical company. FTIR spectra were recorded on a Bruker IFS-55 spectrometer using KBr pellets. SEM images were taken using a Hitachi S- 2400 instrument.
Methods
Polymer synthesis:
Preparation of HDODA-Crosslinked PS: Polymer was synthesized by suspension polymerization method. A mixture of styrene, HDODA, toluene as an inert diluent, and benzoyl peroxide were suspended in a solution of poly(vinyl alcohol) (MW 75,000) dissolved in water. The reaction mixture was kept mechanically stirred at 80°C. The polymerization was completed within 6 h. The beaded product was filtered, washed with hot water, acetone, and methanol and dried. The resin was purified by Soxhlet extraction using acetone. The dried beads were sieved into different mesh sizes and beads of 200-400 mesh sizes were used.
Sulphonation of the copolymer beads: The copolymer beads were sulphonated using conc. sulphuric acid. The sulphonated resin was filtered, washed with distilled water and dried at 60°C for 6 h (Scheme 1).
Scheme 1: Sulphonation reaction pathway [25].
Ion-exchange capacity measurements: The ion–exchange capacity of sulphonated resin was determined by the salt splitting titration. Resin was added into an Erlenmeyer flask containing 2N sodium chloride solution. It was stirred for three hours, and then titrated with standardized sodium hydroxide solution using phenolphthalein as the indicator. The ion- exchange capacity of the sulphonated resin (IEC, meq/g) was calculated from the following equation.
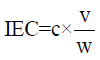
Where ‘c’ is the standardized concentration of sodium hydroxide, ‘v’ is the volume (ml) of the NaOH solution at an end point and ‘w’ is the weight (g) of determined sulphonated resin.
Metal loading to the polymeric support: The polymeric ligand was equilibrated with the Co (II) solution (0.05 M 100 ml) at room temperature for 24 h. The metal loaded polymer was filtered, washed with water and dried under vacuum.
CHNS analysis was done by Perkin-Elmer 2400 Series CHNS analyser. Ion exchange capacity was determined by salt splitting titration. All the resins were characterised by FTIR spectra and surface morphology of PS-HDODA, sulphonated PS-HDODA and metal loaded resin were recorded using SEM.
The copolymer beads were synthesised by suspension polymerisation and then sulphonated using conc. sulphuric acid. The CHNS analysis, FTIR spectra and the ion exchange capacity of the obtained resins were determined. After sulphonation, resins showed swelling in polar solvents indicated the presence of hydrophilic groups in the resin. CHNS analysis (Table 1) gave a sulphur content of 6.27% revealed the attachment of SO3H group (Figures 1-3).
| Sample name | Sulphonation time (min) | Volume of sulphuric acid (mL) | Temperature (°C) | Ion exchange Capacity (IEC) (meq/g) |
|---|---|---|---|---|
| PS-HDODA | 56.11 | 8.14 | Absent | 6.27 |
| SO3H |
Table 1. CHNS analysis.
Tables 2 and 3 shows the ion exchange capacity of sulphonated copolymer beads which were determined by salt splitting titration using standardized NaOH solution. The copolymer beads showed no ion-exchange capacity due to the absence of sulphonic acid group. The sulphonated copolymer showed an ion exchange capacity of 0.94 meq/g.
| Sample name | Sulphonation time (min) | Volume of sulphuric acid (mL) | Temperature (°C) | Ion exchange Capacity (IEC) (meq/g) |
|---|---|---|---|---|
| PS | - | - | - | 0 |
| PS-10S | 10 minute | 2 mL | 60°C | 0.94 |
Table 2. Ion-Exchange capacity of sulphonated resin.
System |
ν (-C=O) cm-1 |
ν (-S=O) cm-1 |
ν (-OH) cm-1 |
ν (=C-H) cm-1 |
ν (M -O) cm-1 |
|---|---|---|---|---|---|
| PS –HDODA resin | 1723 | - | - | 3020 | - |
| Sulphonated PS-HDODA resin | 1715 | 1169 | 3423 | 3194 | - |
| Co (II) loaded sulphonated PS-HDODA resin | 1717 | 1162 | 3440 | 3028 | 695 |
Table 3. Table listing FTIR spectra of Co (II) loaded sulphonated PS-HDODA resin FTIR analysis.
In the FTIR spectrum all the resins exhibited characteristic bands around 1715-1723 cm-1 due to carbonyl stretching frequency. This clearly indicated the formation of polystyrene cross-linked with HDODA. Attachment of sulphonic acid group was confirmed by FTIR spectroscopy. The band around 1169 cm-1 attributed to S=O symmetric stretching vibrations and the band above 3400 cm-1 related to OH stretching frequency in -SO3H group. Metal loading to the resin was confirmed from the frequency 695 cm-1 attributed to metal oxygen stretching frequency. On metal loading the S=O frequency lowered to 1162 cm-1 from 1169 cm-1.
The surface morphology of the polymeric beads were analysed by SEM. These micrographs (Figure 4) clearly showed that the spherical morphology of copolymer beads retained after sulphonation. But the external surface of the sulphonated copolymer beads show scales and cracks probably by the sulphonation process. In fact, even the commercial resins showed defects in their surface after sulphonation.
Application as heterogeneous catalyst: For purification of water from contaminants catalytic approach plays an important role. Here MB decomposition reaction was selected as a model reaction to study the suitability of this resin in water purification. Study showed that polymer supported Co (II) is a potential candidate as catalyst for degradation of MB dye in water. UV- Visible absorbance spectra for degradation of MB dye using the synthesised catalyst is shown in Figure 5. From the plot it is found that, as irradiation time increases, the concentration of MB dye decreases. The plot of percentage efficiency with irradiation time is shown in Figure 6, from which we can say that as irradiation time increases the efficiency of the catalyst also increases.
PS-HDODA copolymer was synthesised by suspension polymerisation. Functionalization of the polymeric support was done by sulphonation using conc. sulphuric acid. The sulphonated PS-HDODA showed ion exchange property of 0.94 meq/g and it is used as ion exchange resin to exchange its counter ion with Co(II) species. Surface morphological analysis of all the resins showed that spherical morphology of the copolymer bead is maintained in all systems. The Co (II) loaded resin showed catalytic activity for the decomposition of MB dye and exhibit a better catalytic efficiency also. It can conveniently used as a novel heterogeneous catalyst system for decomposition of methylene blue in coloured waste water from dye industry and can be used for the purification of waste water from textile industry effluents.
Dr. Jaya T. Varkey acknowledges financial support from UGC in the form of Research Award and Anita Antony is thankful to CSIR India for the financial assistance. We would like to acknowledge SAIF, STIC Cochin for the analysis.
The authors declare that there is no conflict of interest regarding the publication of this paper.