ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
C.Rajeshkannan1 and G.Lakshmanan2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
GCMS is a robust and very widely used technique that combines high sensitivity and specificity for suitable chemical constituents. The identification and quantitation of chemical constituents has been done by GC/MS Ion-trap with respect to the internal and external standard. The internal standards are 60000 patterns of reference compounds and its reference spectrum of NIST library. The present study has been conducted for the quantification of chlorogenic acid (CGA) from the aqueous and methanolic extracts of wood apple (A.marmelos) with external standard by GC-MS Ion trap. Chlorogenic acid is a targeted compound, water soluble phenolic compound it plays vital role in physiological functions in plants and pharmacological activities in humans. The total mass of chlorogenic acid is 354. It would be identified and quantified from different extract of wood apple in comparison with external standard. Retention time of standard chlorogenic acid, aqueous extract, methanolic extract and combined extracts indicates 43.237, 43.236, 43.234 and 43.241 minutes respectively. Mass spectral data of standard fragmented ions are 311.3, 312.3, 325.3, 354.2, 355.0 and these ions were found in all the samples of wood apple. Therefore, quantitation of target compound with respect to the external standard, its similarity of retention time and mass spectral data between the standard and samples would be used for the identification and quantification of target compound. The targeted compound has been quantified as 41.9, 9.5 and 29.7 percent of aqueous extract of WA, methanolic extract of WA and combined extract of WA respectively. GCMS is a selective and sensitive analytical method to identify and quantify the target compound in chemical matrix with reference to internal and external standard of chemical constituents. Target component possess wide application of medicinal properties, especially in psychiatric disorders and this analysis might be used to know the exact concentration of target chemical components in the aqueous and methanolic fruit extract of A.marmelos (wood apple).
Keywords |
| Chlorogenic acid, Wood apple (WA), A.marmelos, target ions, TIC and Mass spectral data. |
INTRODUCTION |
| Phenolic acids are the aromatic secondary plant metabolites widespread throughout the plant kingdom, which is connected with diverse biological function such as protein synthesis, enzymatic activity and also play an important role in the natural host defence mechanism of plant against infectious disease. Cinnamic acid and benzoic acid derivatives are physically dispersed throughout the plants and chlorogenic acid is the most common depside present in plants. Depsides are intermolecular esters which are formed by condensation of two or more molecules of the same or different phenolic acids (Wojciak-kosior and Oniszczuk, 2008). CGA is widely distributed among higher plants (Bradfield et al., 1952; Dickinson and Gawler, 1954) which plays vital role in some physiological functions of plants and pharmacological functions of humans. CGA participates in the defense action of plants (Kirc et al., 1956; Lee and Le, 1958; Uritani and Muramatsu 1953; Uritani, 1953), inhibits IAA oxidase (Gortner and Kent, 1958) and antioxidant properties, which may have positive effects on chronic degenerative diseases (Daglia et al., 2000; Del Castilho et al., 2002). Earlier researchers reported that CGA found to have potential bio-pharmacological importance in humans. A.marmelos commonly known as Bael belongs to Rutaceae Family is widely grown in India, Tropical and subtropical Countries. It possess great mythological and medicinal significance in ancient system of medicine. Many of the researchers reported the presence of number of chemical constituents in A.marmelos viz., γ-sitosterol, aegelin, lupeol, rutin, marmesinin, β-sitosterol, flavone, glycoside, O-isopentenyl halfordiol, marmeline and phenylethyl cinnamamides. Obviously, A.marmelos is a well known traditional medicinal plant in ancient medical system which is proved for its medicinal properties such as antidiabetic, antiulcer, antioxidant, antimalarial, anti-inflammatory, anticancer, radioprotective, antihyperlipidaemic, antifungal, antibacterial and antiviral activities. Enormous methods are available for the quantification of chemical constitutents of botanicals. Gas chromatography /mass spectrometry (GC/MS) has long been used as a method of choice for identifying chemical constituents in complex mixtures and it may fail when acquired spectra are contaminated with extraneous mass spectral peaks as commonly arising for co-eluting compounds, column beed and ion chamber contaminants. However, Stein 1999 reported that compound identification and quantitation has been made by the extraction of spectra from the total ion chromatograms (TIC) and the identification of target compound by the matching of full spectrum from available GC/MS data and trace amount of chemical constituents can also be identified by GC/MS. Therefore, the present study has been conducted for the quantitation of chlorogenic acid in different extracts of A.marmelos with external standard by GC/MS Ion trap. |
MATERIALS AND METHODS |
| Collection of sample |
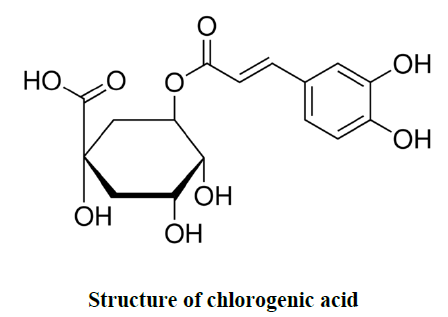
| A. marmelos fruits were collected and authenticated by the Tamilnadu Agricultural University, Coimbatore, Tamilnadu, India. Collected samples were processed and the pulp was dried at 40ºC oven temperature. Then the dried pulp were granulated or powdered by using laboratory blender. The sieved powder was packed in air tight polybags and stored at - 20 ºC for further analysis. Chlorogenic acid (1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid 3-(3,4- dihydroxycinnamate), 3-(3,4-Dihydroxycinnamoyl)quinic acid) was procured from Sigma Aldrich for quantitation in wood apple (A.marmelos). |
 |
 |
| Method of Extraction |
| The fruit powder was extracted sequentially from polar to nonpolar solvents. 100gm of fine powder of fruit pulp was soaked in water for 24hrs followed by methanol and the extracts were filtered, the aqueous and organic filtrate was dried by using spray drier and rotary evaporator respectively. The aqueous phase was dried through spray drier – Lab ultima, Mumbai and the organic phase, excess solvent has been distilled through rotary evaporator and the concentrated organic extract was dried at 40ºC. |
| Sample preparation |
| 50 mg of aqueous, organic and combined extracts of A.marmelos fruit was dissolved in 20 ml of solution (methanol : Benzene : Con. H2So4) in the ratio of 10:11:1 and kept in water bath for 30 minutes. 2ml of n-hexane was added followed by the addition of 10% sodium bicarbonate after 2 min. It was then transferred into a separating funnel, shaken well and left for separation. The organic layer alone was collected while discarding the bottom layer. Clear solution was prepared for injection by passing through C18 –SPE cartridges (Varian Inc, USA). |
| Gas Chromatography and Mass Spectrum (GC/MS) |
| In GC/MS, the analytes are normally derivatized prior to analysis in order to reduce their polarity and facilitate the effective chromatographic separation. It provides a detailed chemical profile of the sample and consequent measurements of relative or absolute amounts of the components. The number of components measured will depend on the resolution of the chromatographic system and the specificity of detection technique. Mass spectrometer is one among the highly specific chromatographic detector. The obtained electron ionization mass spectra are reproducible and suitable for library matching, mass spectral collections being readily available in NIST library. The detection of target compounds has been done, after separation by Gas Chromatography the MS/MS operates by first selecting the target ion(s) of choice at a specific mass during the first stage of MS/MS, which separates the ions from the chemical background or matrix. These selected precursor ions or parent ions are then induced to further dissociate by collision with Helium molecules. The resultant unique product ion spectrum provides confirmation of the target analyte. This increased selectivity of MS/MS also results in an enhancement of the signal to noise ratio; thus somewhat lower limits of detection are achieved. GC/MS/MS provides unequivocal identification in cases where GC/MS spectra of compounds are difficult to interpret. Thus, even if the matrix contains another compound with the same mass as the parent ion for the analyte of interest, it is extremely unlikely that the interfering ion would yield the same daughter ion spectra as the analyte, thus GC/MS/MS is more specific for an ignitable liquid. In order to overcome the pyrolysis product interference and improved detection levels, MS/MS can be utilized as the method of detection. The parent ions and daughter ions are isolated and the MS/MS chromatograms for a variety of hydrocarbon distillates are obtained and subsequently compared to ignitable liquid standards, run under identical conditions. Robert and Eugene (2004) reported that the technique allows the analysis of only one component in the sample. Peak size is plotted against absolute amount of each component or its concentration in the matrix. Techniques of external standardization entail the preparation of standards at the same levels of concentration as the unknown in the same matrix with the known. These standards are then run chromatographically under ideal conditions as the sample. A direct relationship between the peak size and composition of the target component is established and the unknown is either extrapolated graphically or mathematically. |
| Operation condition for GC/M/SMS |
| The GCMS analysis was carried out using a Varian Gas Chromatography (model 3800 series) equipped with Flame ionization Detector and Varian 1079 series injector equipped with Bruker CP 8410 auto sampler, MS transferline temperature of 290ºC. The GC was equipped with fused silica capillary column VF-5ms (30 x 0.25mm). The oven temperature was held at 60ºC for 5 min holding time, then raised up to 160ºC and 290ºC at the rate of 5ºC/min and 10ºC/min respectively. The carrier gas was Helium at a constant flow rate of 1ml / min. The mass spectrometers were operated in full scan mode 40-550 m/z. 1μl of sample was injected at a split ratio of 1:20, MS (Varian 4000 series) analysis was carried out on varian mass spectrometer equipped with NIST library software database. |
RESULT AND DISCUSSION |
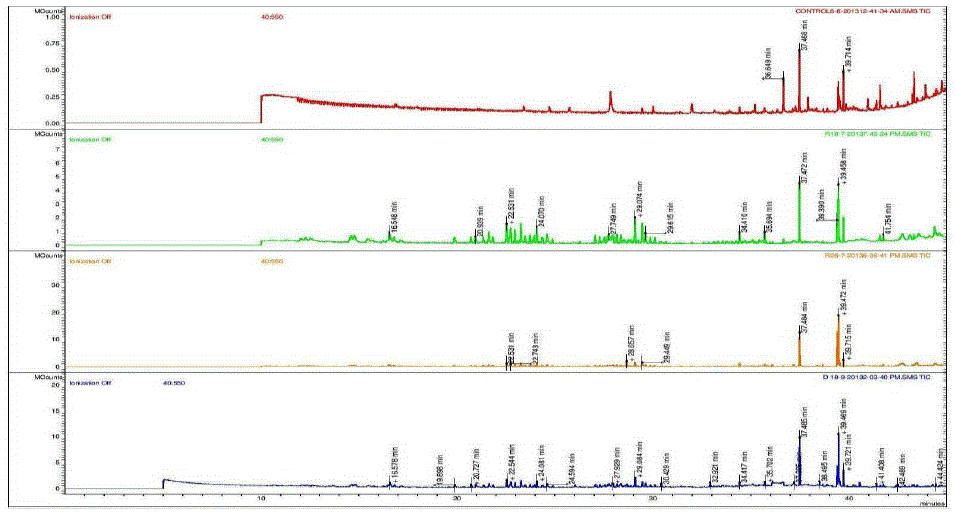
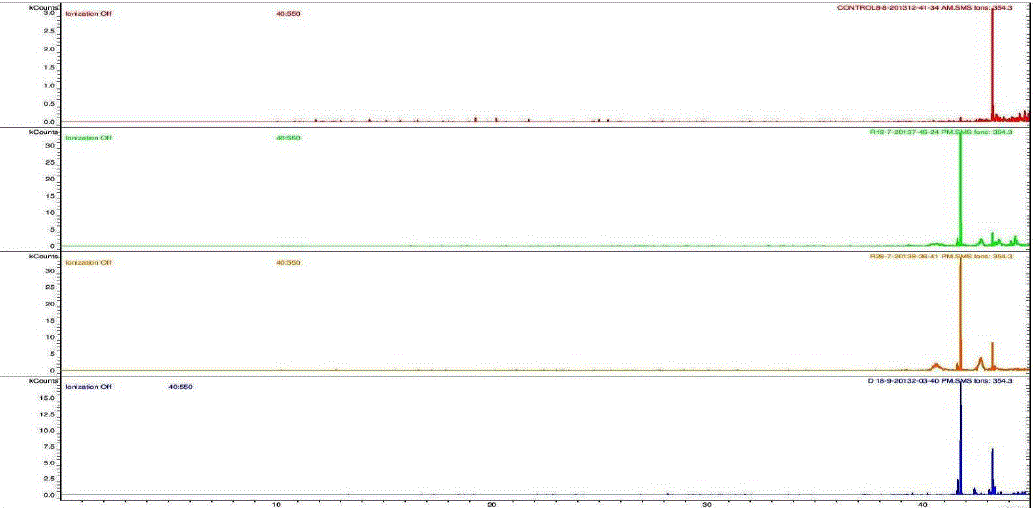
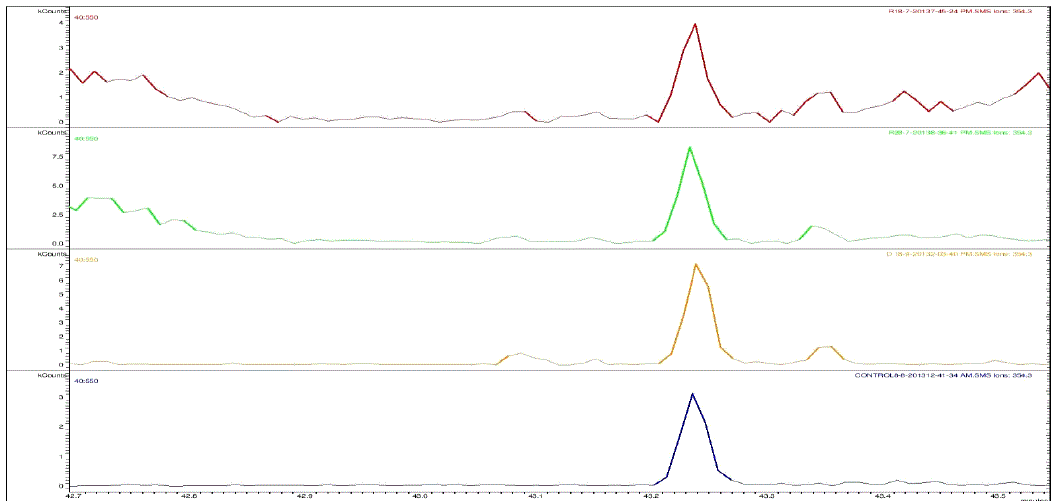
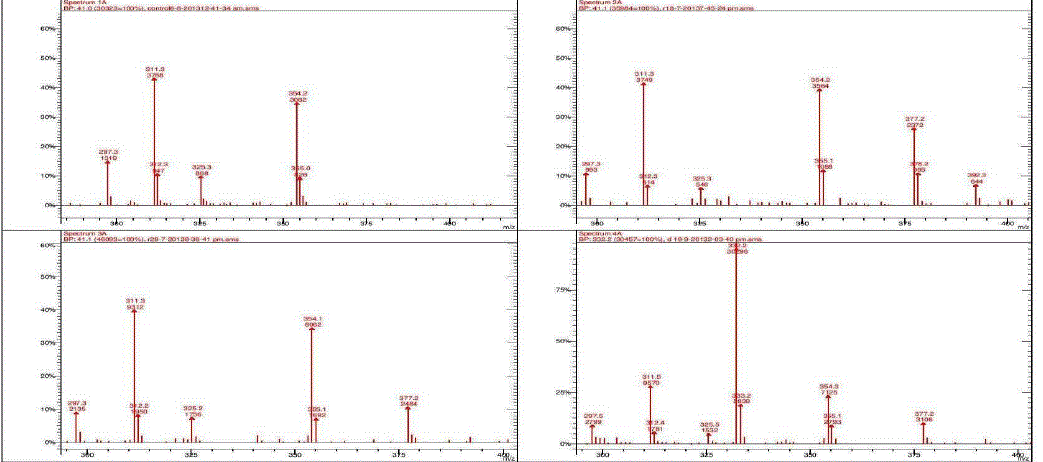
| Nakatani, et al., (2000) reported that the identification and quantitation of three chlorogenic acid in prune fruit by HPLC. GC retention parameters are particularly important. Since retention times vary with the column length, type of stationary phase and temperature, suitable parameters for comparison include relative retention times and retention indices (RI). Relative retention times are simply the ratios of analyte times to the time of a chosen standard compound. Most manufacturers’ data systems do not handle RI values. However, the Automated Mass Spectral Deconvolution and Identification System (AMDIS) (Stein, 1999) from the National Institute of Standards and Technology (NIST) has excellent RI capabilities and is readily available (http:// chemdata.nist.gov/mass-spc/amdis/). The software can read most manufacturers’ data files and perform mass spectral deconvolution in order to ‘clean up’ the mass spectra prior to library searching (www.hdscience.com). The AMDIS software has been applied to plant (Fiehn, 2003). Mass spectrometry data systems are generally equipped for ‘target compound analysis’, where preselected components can be recognized by their mass spectral patterns or pattern of qualifier ions (John et al., 2005). GC/MS/MS is highly sensitive in quantitation of 67 common primary metabolites of which most belong to the groups of amino and nonamino organic acids (Kvitvang et al., 2011). Similarly, the identification and quantitation of chlorogenic acid from aqueous and organic extract of A.marmelos has been done with reference to external standard. GC/MS chromatogram (i) Standard of target compound (Chlorogenic acid) (ii) A. marmelos fruit aqueous extract (iii) A. marmelos fruit methanolic extract and (iv) A. marmelos fruit combined extract was depicted in the Figure 1. Figure 2 represents the peak of the target compound that has been extracted from the total ions of chromatogram (TIC) based on its total mass. The retention times for selected ions in TIC are absolutely similar and length of the peak in X-axis indicates that 0.00 to 0.07 which are depicted in the Figure 3 and Table 1. Mass spectral pattern of target ion in samples and standard has been compared and the fragmented ions were depicted in the Table 2 and Figure 4. |
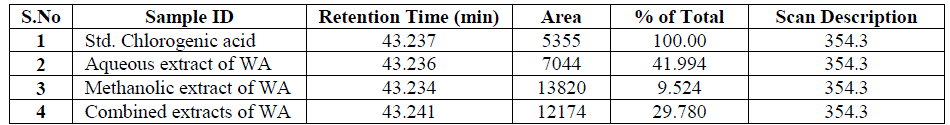 |
| Identification and quantification of chlorogenic acid in different extracts of A.marmelos was depicted in the Table 1 and Table 2 represents the mass data of target ion from total ion chromatogram (TIC). An absolute similarity of retention time and fragmented ions of mass spectral data are used to compare samples with reference external standard chemical compound. Figure 5 indicates the 354.3 ions in TIC and the selected ion has been quantified in percentage of 100, 41.9, 9.5, 29.7 in standard, Aqueous, Methanol and combined extracts respectively. |
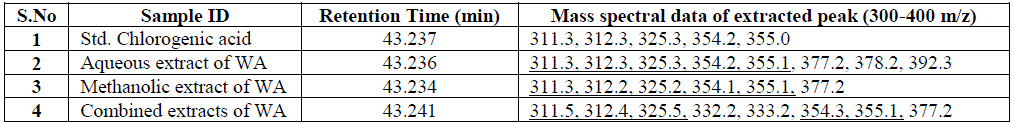 |
| Hites (1992, 1997) reported that the qnantitation of various chemical constituents like di(2-ethylhexyl)phthalate in plasticizers which is well known contaminants and butyl 2-methylbenzoate etc., were identified and quantified based on retention time and its mass spectral data. Similarly the study has been conducted for the identification of target compound chlorogenic acid. Mass spectral data of extracted peak was depicted in Table.2 for comparison between the standard and samples. The total mass of the desired compound has entered for extracting the target compound from TIC. Selected ion and its fragmented ions monitored between the range of 300-400 m/z value. The mass spectral data was observed in standard and samples, which exhibits 311, 312, 325, 354 & 355 mass / charge ratio (m/z) in standard chlorogenic acid and similar m/z values are found in aqueous, methanolic and combined extract of wood apple |
 |
 |
 |
CONCLUSION |
| The present method of study was used for the extraction of target ions from the total ion chromatogram (TIC) and used for the identification and quantitation of chemical components with reference to external standard in GC/MS ion trap. External standard was analyzed in ideal condition as it would be used for the comparison of sample and standard. The selected mass in sample and standard shows the similarities of retention time and mass spectral data. Since, the invention of GCMS played vital role in structure elucidation, identification and quantitation has been done with reference spectrum of NIST and to achieve this some derivatization has been carried out, so that the technique might be expensive. Therefore, the present method could be used for the identification and quantitation of known compounds with external standard from the unknown samples. In order to identify and quantify the targeted chemical constituents, the adopted method would bring the successive results, which are highly specific and sensitive. This technique might be useful for the identification and quantification of targeted chemical compounds and are applicable for further analysis. |
References |
|