e-ISSN: 2319-9849
e-ISSN: 2319-9849
Raafia Najam1 and Syed Muzaffar Ali Andrabi2*
1Department of Chemistry, University of Kashmir, Srinagar - 190006, India.
2University Science Instrumentation Centre, University of Kashmir, Srinagar - 190006, India.
Received date: 07/04/2014; Revised date: 07/07/2014; Accepted date: 22/07/2014
Visit for more related articles at Research & Reviews: Journal of Chemistry
The efficiency of Poplar sawdust as a low cost adsorbent for removing metal ions from aqueous solution has been investigated in this study. Batch experiments were conducted to study the effects of some parameters such as contact time, initial concentration, solution pH and temperature on the adsorption of Cd(II) sawdust. The experimental data were analyzed by the Langmuir and Freundlich models of adsorption and the results fitted well to the Langmuir isotherm. The maximum adsorption capacity of Cd(II) was found 15.15mg/g. Kinetic studies were carried out using the pseudo-first-order and pseudo-second-order models. The experimental data fit very well with the pseudo-second-order kinetic model. Thermodynamic aspects of the adsorption process were investigated. The adsorption of these metals on the sawdust was found to be spontaneous and endothermic in nature.
Heavy metal ions; Adsorption isotherm; Adsorption kinetics; thermodynamics
Heavy metals are frequently present in the aquatic streams by different industrial wastewaters. The existence of heavy metals in aquatic systems can be detrimental to a variety of living species [1]. Heavy metals are not degradable and have increasing significant harmful effect on human physiologies and other biological systems when they exceed the tolerance levels [2]. They pose a significant threat to the environment and public health because of toxicity, incremental accumulation in the food chain and persistence in the ecosystem [3]. Cadmium ions have attracted the attention of researchers as one of the toxic heavy metals and they are found in liquid wastes discharged from a number of industries such as electroplating, dyes and dye intermediates, textiles, tanneries, oil refineries, electroplating, mining, smelters, and so forth. Due to heavy metal contamination in aquatic environment, several episodes increased the awareness about the heavy metal toxicity. Among these, Minamata tragedy due to mercury poisoning and Itali-Itali disease in Japan due to cadmium toxicity are well known. There are various methods for the removal of heavy metals including: chemical precipitation, reverse osmosis, membrane separation, ion exchange [4]. These methods have significant disadvantages, including high energy requirements, incomplete metal removal, generation toxic sludge needs treatment and expensive equipments. Strict environmental protection legislation and public environmental concerns lead the global search for novel and low-cost techniques to remove heavy metals from industrial wastewater [5]. So recent research is directed to developing cost-effective technologies for the removal of metal ions from aqueous solutions.
Biosorption of toxic heavy metals by biomaterials has been suggested as a potential alternative to the conventional methods for recovery of toxic heavy metals from wastewater [6]. Many biomaterials such as walnut hull [7], almond green hull [8], banana skin, green tea waste, oak leaf, walnut shell, peanut shell and rice husk [9], groundnut shell [10], olive stone [11], grape waste [12], hazelnut [13], walnut, hazelnut and almond shell [14], agriculture wastes, carbons [15], rice husk-based active carbon [16], fruit shell of gulmohar [17], coconut husk [18], husk of bengal gram [19], eucalyptus bark [20], agricultural waste biomass[21], pine needles [22], sugar cane bagasse [23], leaf mould [24] and waste pomace of olive factory [25] have been investigated.
The mechanism of sorption on these lignocellulosics substances is complex and includes ion exchange, complexation, chelation, adsorption by physical forces, entrapment in capillaries and spaces of the polysaccharide network and diffusion through cell walls and membranes. There are two main chemical groups that attract and sequester the metals in biosorbent i.e., carboxyl groups in proteins and hydroxyl groups in polysaccharides, apart from amino and carbonyl groups.
The main objective of this study is to access the potentiality of poplar sawdust for the removal of Cd(II) from aqueous solutions. The effect of adsorbent concentration, pH, contact time and temperature on the adsorption capacity was investigated. Adsorption isotherm models and thermodynamic parameterswere also investigated to know the adsorption characteristics.
Adsorbent
The sawdust of poplar tree collected froma local timber workshopwas used as adsorbent in this study. The adsorbent material was washed with deionised water 3 times to ensure that all fine particles are removed. The cleaned material was dried in an oven and was used for adsorption tests.
Chemicals
The stock solution of Cd(II) (1000mg/l) was prepared by dissolving its respective nitrate in double-distilled water. All the chemicals were Analytical Grade reagents from E. Merck India Limited and were used as received. The stock solutions were further diluted with double-distilled water to required concentrations before use.
Adsorption Experiments
Batch adsorption experiments were carried out in stoppered Erlenmayer flasks using the batch equilibrium technique. 1g of sawdust was treated with 100 ml aliquot of the metal ion solution of desired concentration and shaken on a flask shaker for required time interval., After equilibration for the required time interval, the solution was filtered and the filtrate analysed for the metal ion concentration. Metal ion concentration was determined on Atomic Absorption Spectrometer (PerkinElmer Model Analyst-800) housed at USIC, University of Kashmir. In order to obtain the adsorption capacity, the amount of ions adsorbed per mass unit of adsorbent (mg/g) was evaluated using the following expression:
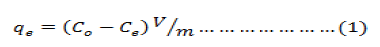
where qe is the amount adsorbed at equilibrium (mg/g), C0 is the initial metal ions concentration (mg/L), Ce is the equilibrium metal ions concentration (mg/L), V is the volume of the aqueous phase (L), and m is the amount of the adsorbent used (g).
SEM analysis of adsorbent
The surface morphology of sawdust was obtained using scanning electron microscope Model Hitachi S3000H (Figure 1). Scanning electron micrographs (SEM) of sawdust was taken before and after adsorption of metal ions. Sawdust exhibits an uneven and rough surface with pores and cavities which increases the surface area available for adsorption of metal ions. Upon adsorption the surface morphology seems to undergo changes due to interaction of the metal ions with donor functional groups on the sawdust.
Effect of contact time
The effect of contact time on the removal of metal ion was studied for a period of 80 minutes (Fig 2). 1g of adsorbent was added to a number of stoppered conical flasks containing 100ml of metal ion solution (100mg/l) and were placed on orbital shaker (120rpm) for each of the different contact times chosen (10, 20, 30, 40.......80minutes). After the given time interval, the mixture was filtered and the filterate analysed for final metal ion concentration on AAS.
The results show that adsorption increases with increase in contact time and reaches equilibrium after 50 minutes at 100mg/l initial concentration of the metal ion. The study revealed that biosorption took place in two steps, a rapid surface adsorption and slow intercellular adsorption. That is probably due to larger surface area of the adsorbent being available at beginning for the adsorption of metals and indicates that initially adsorption of these metal ions is mainly occurring at the surface of the adsorbent. As the surface adsorption sites become exhausted, i.e. at equilibrium, the uptake rate is controlled by the rate at which the adsorbate is transported from the exterior to the interior sites of the adsorbent particles. For all subsequent experiments contact time of 80 minutes was set for the sake of simplicity and to ensure that complete equilibrium is achieved.
Adsorption Kinetics
In order to investigate the mechanism of adsorption, kinetic models are generally used to test the experimental data. The first-order kinetic process has been used for reversible reaction with an equilibrium being established between liquid and solid phases.
The pseudo-first-order kinetic model known as the Lagergen equation is expressed as:-
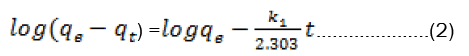
Where qt and qe are the amounts of ion adsorbed at time t and at equilibrium (mg g-1), respectively, and k1is the rateconstant of pseudo-first-order adsorption process (min−1).The slope and intercept of plots of log (qe−qt) versus t were used to determine the first-order rate constant k1 and equilibrium adsorption capacity qe(Fig 3).
The pseudo-second-order kinetic model is given as:
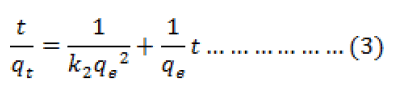
Where k2 is the equilibrium rate constant of pseudo-second-order adsorption (g mg-1min-1). The plot of t/qtversus t gives a linear relationship, and k2 and qe can be calculated from the slope and intercept of the line respectively (Fig 4). The results are given in Table 1. It can be seen that the correlation coefficients (r2) for the pseudo-second-order equation is very close to unity and that the calculated qe values agree well with the experimental ones. These results suggest that the pseudo-second-order model describes the sorption kinetics more appropriately than the pseudo-first-order model. The pseudo-second order model is based on the assumption that the rate limiting step may be a chemical adsorption involving valence forces through sharing or exchange of electrons between adsorbent and adsorbate.
Effect of pH
The effect of pH on the amount of metal ion adsorbed was analyzed over the pH range 1-7. The experiments were not conducted above pH 7 to avoid possible hydroxide precipitation as also reported by Giraldo and Moreno-Pirajan [26]. The pH of the solutions was adjusted to the required value by the addition of 0.1N HNO3 and 0.1N NaOH solutions
pH is an important parameter affecting the adsorption of metal ions on biosorbent because it affects metal speciation in solution as well as influences the surface properties of the adsorbents in terms of dissociation of functional groups and surface charge. Fig. 5 shows the effect of pH on adsorption of Cd(II). The result shows that adsorption of Cd (II) increases as pH increases from 2-4 and after that adsorption remains constant. As the pH of the system increases, the number of positively charged sites decreases and the number ofnegatively charged sites increases on the surface of adsorbent and thus favours the adsorption of Cd(II) due to electrostatic attraction. Cd species are found to be present in aqueous solution in the form of Cd2+, Cd(OH)+, Cd(OH)2 , Cd(OH)2 (s), etc. Cd2+ is the only ionic species present in the solution at pH<6. In alkaline range, precipitation plays the main role in the removal of Cd2+ which is attributed to the formation of Cd(OH)2(s). For all subsequent experiments, a pH of 4 was selected in order to ensure that Cd(II) doesnot precipitate as hydroxide.
Effect of Metal ion Concentration (Adsorption Equilibrium)
The effect of metal ion concentration on the adsorption was studied by shaking 1g of adsorbentwith 100 ml aliquots of metal ion solutions of different initial concentrations (20-200mg/l) for 80 minutes. After equilibration for 80 minutes, the supernatant solutions were filtered and the filtrates analysed for the final metal ion concentration.
The effect of initial concentration (20-200mg/l) of Cd2+ on its adsorption on sawdust from aqueous solution was investigated. The results, presented in Figures 6 show that adsorption starts from a low concentration, and with an increase in the metal ion concentration the amount of metal ion adsorbed increases. At low concentrations, metal ions are adsorbed at specific sites and with increasing metal concentrations the binding sites become more quickly saturated as the amount of adsorbent remained constant. That is, there is some metal concentration that produces the maximum adsorption for a given mass of adsorbent and thereafter, adding more metal cannot increase adsorption because no more sites are available.
Adsorption isotherm
Adsorption isotherm describes the equilibrium relationships between adsorbent and adsorbate. The Langmuir isotherm is valid for monolayer adsorption onto a surface with homogeneous adsorption sites. The linearized form of the Langmuir equation can be written as
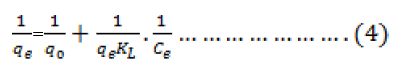
Where qo and KL are Langmuirparameters related to maximum adsorption capacity and free energy of adsorption respectively. Ce is the equilibrium concentration in the aqueous solution and qe is the equilibrium adsorption capacity of adsorbent. Figure 7 represent the Langmuir isotherms of the mentioned metal ion.
The Freundlich model is an empirical equation based on adsorption on heterogeneous surface. The linearized form of the Freundlich isotherm can be written as
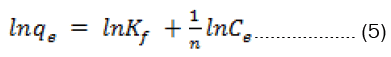
Where Kf and n are the Freundlich constants that indicate adsorption capacity and adsorption intensity, respectively. The Freundlich constants, Kf and 1/n, can be determined from the intercept and slope of linear plot of ln qe versus ln Ce, respectively (Figures 8).
The isotherm constants and correlation coefficients are given in table 2. From the plot it is clear that the adsorption equilibrium data fitted the Langmuir as well as the Freundlich equations well with good correlation coefficients. However, the adsorption equilibrium data for Cd2+ fitted the Langmuir isotherm equation with correlation coefficient of 0.996 as compared to 0.978 for Freundlich isotherm equation and thus indicating the best fit of the adsorption equilibrium data in the Langmuir isotherm than in case of Freundlich isotherm.
Effect of temperature
The effect of temperature on the adsorption was studied at three different temperatures of 20o, 30o and 40oC by shaking 1 g of adsorbentwith 100 ml aliquots of metal ion solutions of a particular concentration (100mg/l). The mixture was heated and shaken to the appropriate temperature by using temperature controlled flask shaker. At a particular temperature, the supernatant solutions were filtered and filtrates analyzed for the final metal ion concentration.
The variation of adsorption with temperature was studied. Figures 9 show the adsorption of metal ions on sawdust increases as temperature increases. The increase in adsorption capacity with temperature suggested that the active sites available for adsorption have increased with temperature. This could also be attributed to the pore size variation and enhancing rate of intraparticle diffusion of solute since diffusion is an endothermic process.
In order to explain the effect of temperature on the adsorption of metal ions on sawdust, thermodynamic parameters: Gibbs free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) were obtained by using the following equations
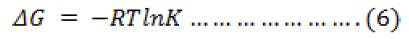
Where R is the ideal gas constant  and T is the temperature (K).
and T is the temperature (K).
The enthalpy and entropy of adsorption were determined from the Van’t Hoff equation
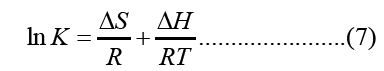
Where, ΔH and ΔS were obtained from the slope and intercept of the Van’t Hoff’s plot of ln K verses 1/T as shown in Figure 10.
The results of the thermodynamic calculations are shown in table 3. The negative value of ΔG indicates the feasibility of the adsorption process and high affinity of the metal ion towards the adsorbent. A positive value of ΔH confirms the endothermic nature of the process. The positive value of ΔS shows the increased randomness at solid/solution interface during the adsorption process. The adsorbed water molecules, which are displaced by the adsorbate species, gain more translational energy than is lost by the adsorbate ions, thus allowing the prevalence of randomness in the system. The enhancement of adsorption at higher temperature may be attributed to the enlargement of pore size and/ or activation of adsorbent surface.
This study clearly shows that sawdust which is cheap, natural and abundant material can be used as an effective adsorbent for the removal of heavy metal ions from aqueous solution. Various parameters affecting the adsorption process like contact time, pH, initial metal concentration, and temperature were studied. The thermodynamic parameters like ΔH, ΔS and ΔG were calculated and the positive values of ΔH and negative value of ΔG shows that the adsorption of these metal ions on sawdust is endothermic and spontaneous in nature. The equilibrium adsorption data were analysed by the Langmuir and Freundlich isotherm models but the best fit was obtained with the Langmuir isotherm. The kinetic equilibrium data fits the pseudo-second order kinetics, thus suggesting chemisorption as the rate determining step in the adsorption of these metal ions on sawdust.
The authors are highly thankful to UGC for providing financial support in the form of Research Project.