ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
PaliShahjee1, B.J.Godboley2, A.M.Sudame3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Fluoride contamination in drinking water due to natural and anthropogenic activities has been recognized as one of the major issue imposing a serious threat to human health. Among several treatment technologies applied for fluoride removal, adsorption process has been explored widely and offers satisfactory results, so objective of this study was to investigate or check efficiency of low cost adsorbent (Bleaching Powder) for the removal of excess fluoride from aqueous solution. The influence of various operational parameters viz. effect of adsorbent dose, pH, initial concentration and contact time were studied by a series of batch adsorption experiments. 7.3gm/100ml (0.7mg/l is the new limit of fluoride according to EPA & HSS) was calculated as optimum dose of adsorbent. It was noticed that adsorbent is showing a good efficiency between 6-10 pH. In contact time variation it was noticed that it show rapid adsorption of Fluoride from 4 to 7 hr, thereafter, the adsorption rate gradually reaches equilibrium in 8hr-9hr. These conditions make it very suitable for use in drinking water treatment. From these studies, it may be concluded that bleaching powder is an efficient and economical adsorbent for fluoride removal from aqueous solutions.
Keywords |
| Bleaching powder; fluoride removal; sorption; adsorbent dose. |
INTRODUCTION |
| Fluoride-related health hazards are a serious environmental problem across the globe. They can occur due to both natural as well as anthropogenic activities. Since fluoride is present in several naturally occurring minerals and geochemical deposits, it can be leached out by rainwater, thereby contaminating ground and surface water. On the other hand, several fluoride containing compounds have industrial applications, are extensively used in industries such as semiconductors, fertilizers, and electrolysis of alumina, and contribute to fluoride pollution. Drinking water containing fluoride may be beneficial or detrimental depending upon its concentration and total amount consumed [1]. Over the past few decades, scientific literature in fluoride research suggests that fluoride concentrations between 0.7–1.2 mg/L are beneficial, especially to infants for the prevention of dental caries or tooth decay, but concentrations above1.2 mg/L cause mottling of teeth in mild cases but fluorosis (dental or skeletal) and several neurological disorders in severe cases [2, 3]. Increasing fluoride concentration in ground water has already become a serious problem across the globe. India is among 23 countries in the world facing this chronic disease. It is estimated that around 62 million people in 19 states of India are affected with various forms of fluorosis, which include dental, skeletal and non-skeletal manifestations [4]. Taking health effects into consideration, the EPA & HSS has given a guideline value of 0.7 mg/L as the lower limit of fluoride in drinking water [2]. Excess fluoride is generally encountered in ground water, and more than 80% of the rural and 50% of the urban population in India depend on ground water for domestic needs [5]. Consequently, it becomes essential to bring down the fluoride concentration within the permissible limit of 0.7 mg/L according to EPA & HSS [2,6]. Defluoridation is generally carried out by adsorption, chemical treatment, electrochemical methods, dialysis and the ion-exchange process [7, 8]. Among them, adsorption is found to be effective, environmentally friendly and economical [9]. Recently, scientists are mostly focusing their study on various types of inexpensive and effective materials such as different clays, spent bleaching earths, activated alumina, coconut shell carbon, chemically activated carbon, bone charcoal, natural zeolites, burnt clay and other low cost adsorbents with various degrees of success [10, 11]. So, there is an urgent need to explore low cost & locally available defluoridation materials for safe and easy use at both household and small community levels. Bleaching powder also known as chloride of lime, or chlorinated l ime; calcium oxychloride is a white or mostly white powder that mainly consists of calcium hypochloride. It is widely used as a disinfectant for drinking or swimming pool water and also as bleaching agent [12, 13]. The main advantage of using bleaching powder for fluoride removal over other chemical treatment methods is that, along with being a disinfectant, it also acts as a defluoridation agent. So, the main aim of this study was to investigate the fluoride removal efficiency of bleaching powder by batch adsorption studies. |
II. EXPERIMENTAL |
| II.1 Materials:All chemicals used in the present study were of analytical reagent grade. Bleaching powder (CaOCl2) utilized as adsorbent was obtained from locally manufactured commercial sources. Sodium fluoride (NaF), sodium hydroxide (NaOH) & hydrochloric acid (HCl) was purchased from E-Merck (India), Mumbai. A stock solution (100 mg/L) of fluoride was prepared by dissolving 221 mg of anhydrous NaF in 1 L of distilled water and the desired working fluoride solution was prepared from stock solution by appropriate dilution. |
| Adsorption experiments: All experiments were carried out with a constant volume (100 ml) of the desired fluoride solution (5 ppm) along with the known weight of adsorbent in 250 ml Tarson conical flasks on a orbital shaking incubator at room temperature (27±3°c) to study the influence of various operational parameters. At the end of the desired contact time, the samples were filtered by using Whatman filter paper No. 42 and the filtrate was analyzed for residual fluoride concentration. The effect of solution pH on fluoride removal was studied by adjusting the pH of the solution by using 0.1 N HCl or 0.1 N NaOH. A similar procedure was followed to determine the optimum conditions and to study the influence of various parameters such as pH, initial fluoride concentration, adsorbent dose etc. The fluoride loading capacity, qe(mg/g) was obtained by using the following equation (1) |
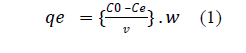 |
| Where C0 and Ce(both in mg/g) are the initial and equilibrium fluoride concentration respectively, V (L) is the volume of the aqueous solution and W (g) is the mass of the adsorbent. |
| II.3Methods of analysis: After attaining equilibrium, the experimental samples were filtered through Whatman filter paper No. 42 and total ionic strength adjusting buffer solution was added to samples in the ratio 1:10 before evaluation. This solution regulates the ionic strength of samples & also adjusts the pH within a measurable range & reduces the free fluoride concentration in the solution. Then, the resultant solution was analyzed for residual fluoride concentration by using fluoride an ion selective electrode. A similar procedure was used for the experiments on the effect of pH, initial concentration, etc. |
| II.4Physical characterization: The bleaching powder before (untreated) and after (treated) batch adsorption was characterized using SEM (Scanning electron microscopy). A representative experiment was carried out under the given conditions: initial fluoride concentration = 5 mg/L, temperature = 30ºC, shaking speed = 150 rpm and contact time = 24 h respectively, for the preparation of SEM samples. The SEM analysis was carried out in IIT Delhi Lab. |
III. RESULTS AND DISCUSSION |
| When bleaching powder is added to water, it is converted into lime [Ca(OH)2], chloride and hypochlorite ions [14]. |
| CaOCl2 → Ca2+ + Cl- + OCl- |
| A part of the Ca(OH)2 goes into the solution and the remaining is left as solid. The portion which dissolved into the solution reacts with fluoride to produce calcium fluoride (CaF2), which is insoluble and is left as precipitate. The major portion of Ca(OH)2 does not go into the solution. So, there is adsorption of fluoride by a surface chemical reaction in which hydroxide ions of Ca(OH)2 are replaced by fluoride ions with the formation of CaF2 [15] Ca(OH)2 + 2F- → CaF2 (s)+ 2OH- This suggests that fluoride removal by bleaching powder mostly takes place by adsorption (i.e. chemisorptions) and slightly due to precipitation in the form of calcium fluoride. This was also explained by taking SEM of the bleaching powder, before and after the adsorption process (Fig. 1a and 1b). The SEM images of the bleaching powder before treatment of fluoride ions shows cluster of very fine particles with a surface coating while the SEM images of the bleaching powder after treatment of fluoride ions shows small irregularly shaped fine particles—mostly fluoride ions—adhering to the surfaces of the adsorbent. Fig. 1 |
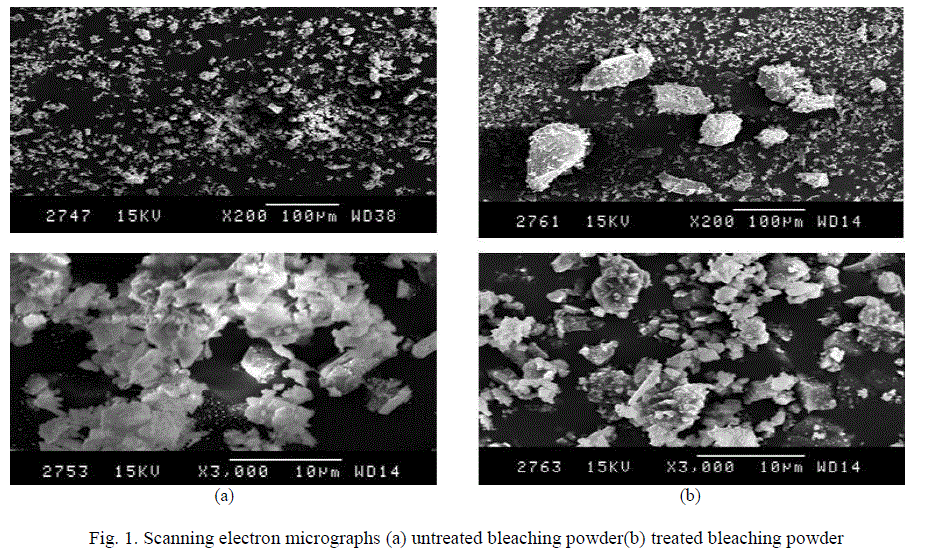 |
| Fig. 1. Scanning electron micrographs (a) untreated bleaching powder(b) treated bleaching powder |
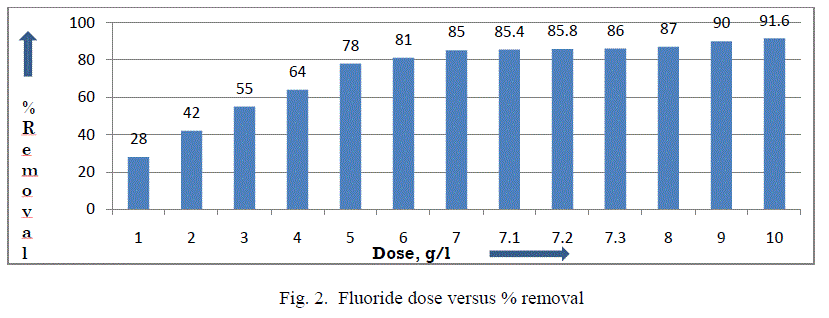
| III.1 Effect of adsorbent dose: The influence of adsorbent dose on fluoride removal is checked at a fixed initial fluoride concentration of 5 mg/L, pH 6-7 at temperature 27±3°C. It was noticed that percentage removal of fluoride increased from 28% to 90.60% with an increase in adsorbent dose from 10 g/L to 100 g/L respectively. However, it was noticed from fig. 2 that after a dosage of 73 g/L, there was no significant change in the percentage removal of fluoride and also it was required to bring down the fluoride level below 0.7mg/L as EPA & HSS. It may be due to the overlapping of active sites at higher dosage, thus decreasing the net surface area of the adsorbent [11, 16]. So, an adsorbent dose of 73 g/L was used for further study. |
 |
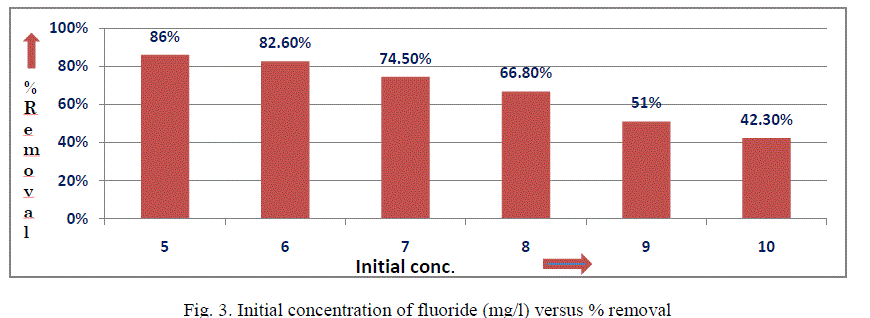
| III.2 Effect of initial concentration:The influence of initial fluoride concentration on fluoride removal from aqueous solution was studied by keeping all other parameters constant (i.e. dose = 73 g/L, pH = 6-7 and contact time =24h) as shown in Fig. 3. It was noticed that with increase in initial fluoride concentration, the percentage removal of fluoride decreases while the loading capacity increases. This suggests that there exists a reduction in immediate solute adsorption due to the lack of available active sites on the adsorption surface, compared with the relatively large number of active sites required for the high initial concentration of fluoride. |
 |
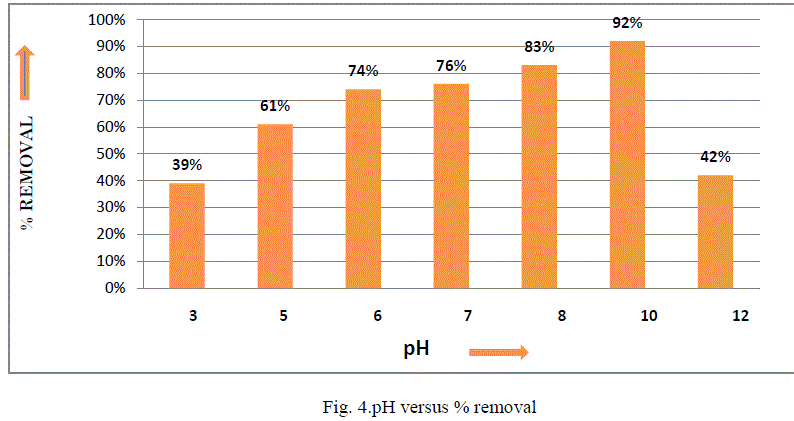
| III.3 Effect of pH: The pH of a solution plays an important role in the uptake of fluoride from aqueous solution on bleaching powder. The fluoride removal efficiency of bleaching powder was investigated over a pH range of 3–12 with an adsorbent dose of 73 g/L, initial fluoride concentration 5 mg/L, shaking speed 150 rpm and contact time 24 h as shown in Fig. 4. It was evident from the results that bleaching powder exhibited reasonably significant fluoride removal over a pH range of 6–10. The lower fluoride removal in acidic medium might be due to the formation of weakly ionized hydrofluoric acid, which reduces availability of free fluoride for adsorption [11]. Similarly, in highly alkaline conditions, lower fluoride removal may be due to the formation of Ca(OH)2, which subsequently reduces the availability of calcium [17]. |
 |
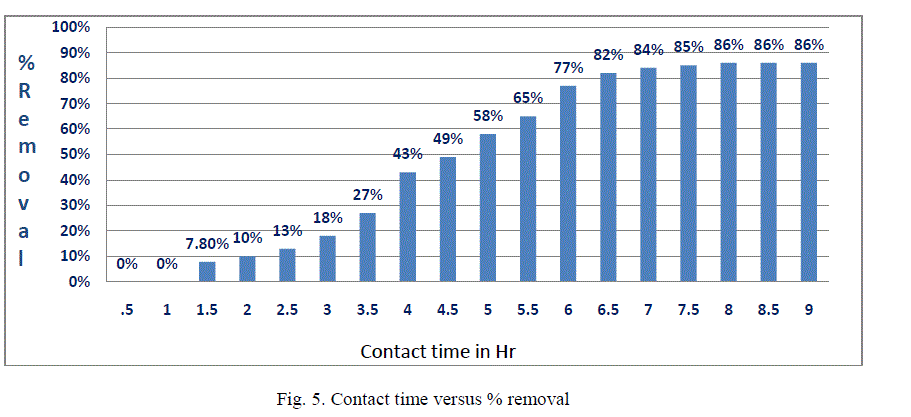
| III.4 Effect of contact time: The effect of contact time on the removal of fluoride by Bleaching powder was studied. The contact time curves fig. 5 shows rapid adsorption of Fluoride from 4 to 7 hr, thereafter, the adsorption rate gradually reaches equilibrium in 8hr-9hr (optimum contact time).Aggregation of Fluoride molecules with the increase in contact time makes it almost impossible to diffuse deeper into the adsorbent structure at highest energy sites. This is because the mesopores get filled up and start offering resistance to diffusion of aggregated fluoride molecules in the adsorbents. |
 |
IV. CONCLUSION |
| The results obtained the present work demonstrate that bleaching powder generally has advantageous characteristics as an economical and viable substitute for other adsorbents. One of the major advantages of using bleaching powder over other chemical treatment methods is that, along with being a disinfectant, it also acts as a defluoridation agent. This study concludes the following: 1. Optimum dose of adsorbent was found 7.3gm/100ml for removal of fluoride of 5ppm concentration. 2. Adsorption capacity is more when pH is about 10. 3. Optimum time was found to be 8hrs Scope of future work:On the basis of the results; model type filter which will be eco-friendly as well as economical for the rural people can be developed. |
References |
|