ISSN ONLINE(2278-8875) PRINT (2320-3765)
ISSN ONLINE(2278-8875) PRINT (2320-3765)
Ramesh Khosla1, Renuka Choudhary1, Nayan K Tiwari1, Dinesh Chandra2, Ajeet Kumar Yadawa3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering
In order to meet the increasing demand of power sources for portable electronic gadgets, researchers have devoted significant attention towards new ambient energy sources. To run the device for longer period in operation and standby times, we require some external energy sources to charge the batteries from time to time. Mobile batteries are generally charged by conventional method i.e. step down mechanism followed by rectification and filtering. This process causes different types of losses which results in inefficient utilization of power. This paper discusses developments taking place in the field of batteries, energy harvesting and charging techniques to create efficient power system for portable and mobile devices. This paper presents some of ambient energy sources which are naturally present in the environment which can be utilized in charging. In addition to this, special types of materials for batteries to increase its power capacity are also discussed.
Keywords |
| Batteries, electrochemical processes, energy harvesting, portable device, graphene, li-ion batteries, piezoelectric transducers, magnetic resonance, mobile, carbon nanotubes, nanomaterials piezoelectricity, organic radical batteries, power conversion, solid electrolyte interphace (SEI), Si anode, solar energy, wireless power transmission(WPT). |
INTRODUCTION |
| In last decade we have seen electronic devices shrink in volume. Due to internet connectivity we carry world’s knowledge in our pocket. Touch based tablet make computing intuitive enough for even two year olds. Everything seems to improving well but one big exception, batteries. Smartphone clock speeds are measured well in GHz and screens are so dense that we can’t even see pixels everywhere, battery life is still measured in hours and not in days. A number of compromising developments on the horizon promises to bring batteries out of the dark ages and potentially last for a weeks even for 4G services within reach. |
| The large amount of energy consumption and increasing mobility of electronic devices force us to demand new power sources. This paper deals with the following aspect of the development in these areas: |
| (1) Battery Technologies |
| (2) Energy Harvesting Techniques |
| (3) Charging Techniques |
| These technologies and techniques help us to develop new efficient batteries, self power generating electronic gadgets and chargers. |
LITREATURE REVIEW |
| In the 1960s Gordon Moore predicted - “The number of transistor incorporated in a chip will approximately double every 24 months” [1]. This is also offering two times processing power at least each second year but development in batteries which did not even double over last decade. So there is a need of other sources of energy and long running batteries because of ever increasing applications in mobile phones. Although there have been technological advancement in battery technology over the years, but noteworthy outcome has not yet emerged. |
| This paper presents some newly developed material for cathode and anode, which help in improving the battery capacity and longevity. |
(1) Battery Technologies |
| Presently, Li-ion (Lithium-ion) batteries have been widely used in portable electronic gadgets. Li-ion battery is offering a higher energy density than NiMH (Nickel Metal Hydride) battery. In addition, these batteries allow for a large number of charge/discharge cycles without memory effect which ensures a long battery life time [2]. But, the energy density and performance of Li-ion batteries is largely depends on the physical and chemical properties of cathode and anode material. There are different types of Li-ion batteries and the difference lies mainly in the cathode materials For e.g. Lithium Cobalt Oxide, Lithium Manganese Oxide, Lithium Iron Phosphate etc. Some new materials are also appearing in the anode to replace graphite (not only anode but also cathode). This review paper discusses the researches in the past decade of different cathode and anode materials. Some of them are mentioned below: |
| (a) Lithium -air battery |
| (b) Si nano particles as anode for Li-ion battery |
| (c) Sulfur as cathode for Li-ion battery |
| (d) Lithium Lanthanum Titanium Oxide (LLTO) coated cathode battery |
| (e) Lithium polymer battery |
| (f) Organic radical battery |
| (g) Green rice Li –ion battery |
| (h) Graphene as anode for Li-ion battery |
| a) Lithium - air battery: This type of battery uses Li metal as anode and oxygen as cathode. The superior energy density offered by lithium-air batteries is due to the coupling of a lithium anode to atmospheric oxygen through a porous carbon-based air cathode, instead of the heavy conventional compounds found in lithium-ion batteries. During battery discharge, lithium ions flow from the anode through an electrolyte and combine with oxygen at the cathode to form lithium oxides, which are inserted into the cathode. During recharging, the lithium oxides separate again into lithium and oxygen, hence the process can begin again. The carbon-based electrodes used in last year's research had only about 70 percent void space, but the new carbon-fibre-based electrodes are significantly more porous and boast more than 90 percent void space. This means the carbon-fibre-based electrodes can more efficiently store the lithium oxide that fills the pores as the battery discharges. The carbon-fiber-based electrode can store four times more energy for its weight compared to current lithium-ion battery electrodes [3]. The main challenges are practical implementation of this battery is to keep the battery protected from the environment as atmospheric oxygen consists of humidity which degrade cathode [4]. |
| b) Si nano particles as Anode for Li-ion battery: This type of battery use Li metal as cathode and Si as anode. The electrochemical alloying reaction of Li with Si involves volume expansion of up to 400% during lithium insertion, and upon extraction of the lithium involves significant contraction. Stress induced by the large volume change causes cracking and pulverization of silicon, which leads to loss of electrical contact and eventual capacity fading. Another challenge for silicon anodes is the unstable solid electrolyte interface (SEI). The repetitive volume expansion and contraction constantly shifts the interface between Si and the organic electrolyte, hence preventing the formation of a layer of stable SEI, which in turn results in a low Columbic efficiency and a decrease in capacity during battery cycle. Silicon anodes should also maintain good electrical contact between Si materials and current collector during cycling. Even though mechanical fracture does not take place in Si nanostructures below critical sizes, large volume change can still cause the movement of Si nanostructures and the detachment from the conducting environment during long term battery cycling. Finally, these challenges are compounded with the fact that the synthesis of these silicon anodes must be cheap and scalable to supplant current carbon anodes in Li-ion batteries [5]. |
| c) Sulfur as cathode for Li-ion battery: In these type of batteries, sulfur cathode is used, which was capable of storing five times more energy than other commercially available batteries. But sulfur particle suffer from the following problems: |
| i. Poor electronic conductivity |
| ii. Dissolution of intermediate polysulphide |
| iii. Large volumetric expansion, approximately 80%. |
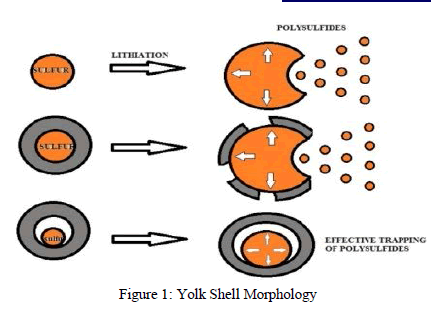 |
| This problem can be solved by encapsulating sulfur with porous carbon or graphene oxide or Sulfur –TiO2 yolk shell (a nano architecture which is a nano particle that is made up of an inner core of sulfur, surrounded by an outer layer of porous Titanium oxide) [20].This morphology provides internal void space to accommodate the volume expansion of sulfur during lithiation, resulting in a structurally intact shell for effective trapping of polysulfide’s, respectively [6]. |
| d) Lithium Lanthanum Titanium Oxide (LLTO) coated cathode battery : In terms of material researches for batteries cathode and anode material, researchers found a very useful compound Li−La−Ti−O (LLTO), which is a fast lithium-ion conductor acts as an effective coating material for cathode materials used in rechargeable lithium-ion batteries. This fast Li-ion conductor is characterized by first-principles calculations showing low activation barrier for lithium diffusion at various different lithium concentrations. The possible reasons for such enhancements are explored. Experimentally using potentiostatic intermittent titration technique (PITT), electrochemical impedance spectroscopy (EIS), due to high lithium conductivity in the LLTO coating material, the chemical Li+ diffusion coefficient is one magnitude higher in the coated samples than that in the uncoated samples. In addition, the impedances of both interfacial charge transfer and Li+ transportation in the solid-electrolyte-interphace (SEI) layer are reduced up to 50% in the coated samples [7]. |
| e) Lithium Polymer battery: Li polymer batteries are usually composed of several identical secondary cells in parallel to increase the discharge current capability, and are often available in series "packs" to increase the total available voltage. The primary difference is that the lithium salt electrolyte not held in an organic solvent in a solid polymer composite such as polyethylene oxide or polyacryonitrile. The advantages of Li-ion polymer over the lithium-ion design include potentially lower cost of manufacture, adaptability to a wide variety of packaging shapes, reliability, and ruggedness, with the disadvantage of holding less charge. Lithium-ion polymer batteries started appearing in consumer electronics around 1995 [8]. |
| f) Organic radical battery: An organic radical battery (ORB) is a relatively new type of eco friendly, high power, high energy density rechargeable battery was first developed in 2005[9]. It uses an organic radical polymer, which is a flexible plastic, instead of metal to provide power. The polymer contains stable radicals, which give its unique properties, and takes the form of a gel saturated with electrolytes. |
| Organic radical batteries are composed of stable organic radical polymers, for example poly(2, 2, 6, 6- tetramethyl piperidine-N-oxyl- 4ylmethacrylate) (PTMA) as a cathode active material [9]. The anode, which is the negative side of the battery, is made of the same carbon used in rechargeable lithium-ion batteries. The cathode of an ORB is highly conductive and created with a gel made of organic radical solids and carbon fibres, permeated with electrolytes [9]. |
| g) Green rice Li –ion battery: In latest technological incarnation, the climbing herb could lay the foundation for an ecofriendly alternative to traditional lithium-ion (Li-ion) batteries. Production and recycling pumps produces an estimated 72 kilograms of carbon dioxide, a greenhouse gas, into the atmosphere for every kilowatt-hour of energy in a Li-ion battery [10]. These grim facts have fed a surging demand to develop green batteries. Biologically based colour molecules, like purpurin (1,2,4-Trihydroxyanthraquinon) and its relatives, seem pre-adapted to act as a battery's electrode. In the case of purpurin, the molecule's six-membered (aromatic) rings are festooned with carbonyl and hydroxyl groups adept at passing electrons back and forth, just as traditional electrodes do. These aromatic systems are electron-rich molecules that easily coordinate with lithium. Moreover, growing madder (A southwest Asian perennial Plant) or other biomass crops to make batteries would soak up carbon dioxide and eliminate the disposal problem, without its toxic components, a lithium-ion battery could be thrown away [11]. |
| h) Graphene as anode for Li-ion battery: Graphene is the two dimensional sheet of sp2 hybridized carbon, extended by honeycomb network is the basic building block of other important allotropes: it can be stacked to form 3D graphite, rolled to form 1 D nanotubes, and wrapped to form 0D fullerenes. So 1D nanotubes and 0D fullerenes have been used as anode material in Li-ion batteries and exhibit improved electrochemical performances. High quality graphene sheets with curled morphology consisting of a thin wrinkled paper like structure, fewer layers (4 layers) and a large specific area (492.5 m2g-1) were prepared using a thermal exfoliation method. The electrochemical performance testing showed that the first reversible specific capacity of the prepared graphene sheets was as high as 1264 mAhg-1 at a current density of 100 mAg-1. After 40 cycles at different current densities of 100 mAg-1, 300 mAg-1, 500 mAg-1 and 1000 mAg-1 and the reversible capacity was still maintained at 848 mAg-1 , which indicated that the prepared graphene posses a good cycle performance for lithium storage [12]. |
| On the above discussions a comparison chart is given below. |
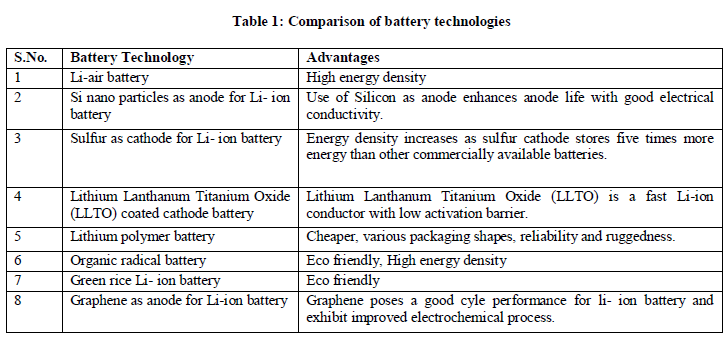 |
(2) Energy Harvesting Techniques |
| Energy harvesting is the process by which energy is derived from external sources e.g. solar energy, thermal energy, wind energy, salinity gradients and kinetic energy captured and stored for small, wireless autonomous devices, like those used in electronic systems and wireless sensor networks. |
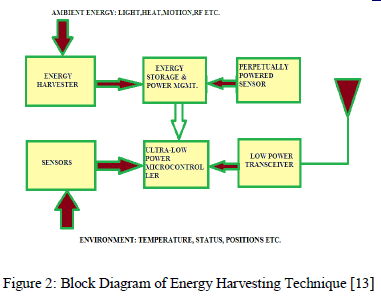 |
| Energy harvesters provide a very small amount of power for low-energy electronics. Out of many abundant energy sources which may use to harvest energy for electronic devices, some more relevant techniques are mention below. |
| (a) Power from human breathing |
| (b) Power from people’s fat |
| (c) Power from blood pressure |
| (d) Power from Typing Keyboard |
| (e) Charging from shoes |
a) Power from human breathing: |
| An average person of 68 kg has an approximate air intake rate of 30 liters per minute. However, available breath pressure is only 2% above atmospheric pressure. Studies indicate that the power consumed by pulmonary ventilation (breathing) is between 0.1 and 40 Watts. Increasing the effort required for intake of breath may have adverse physiological effects so only exhalation will be considered for generation of energy. Thus, the maximum available power is: |
| W=P X ΔV |
| P= 0.02 X 1.013 X 10^5 Kg/msec^2) (30 L / 1min) X (1 min /60 sec) X 1 m^3/1000 L) =1.0 watts/minute Researchers have explored the way of tapping the energy from breathing of powering implantable electronics. In this way researchers put experiments on dog and they extracted17 μW and with improvement claim to be able to attain 1mW [14]. |
b) Power from blood pressure: |
| Portable electronic gadgets can also be charged by emerging generated from blood pressure. For e.g. Average blood pressure of 100 mmHg( 120/80 mmHg), a resting heart rate of 60 beats per minute and a heart stroke volume of 70 ml passing through the aorta per beat, then the power generated is, P = (100mmHg) X (1.013X 10^5 Kg/msec^2) / (760 mmHg) X (60 beats/1 min) X (1 min/60 Sec) X 0.071 X (1m^3/1000) = 0.93 watts [14]. |
| c) Power from people’s fat: The human body is tremendous storehouse of energy. One gram of fat stores approx. 9000 calories. For e.g. an average man of 68kg with 15% body fat stores energy approximately equivalent to 384 MJ. Similarly, Table 1 provides a perspective on the amount of power used by the human body during various activities: |
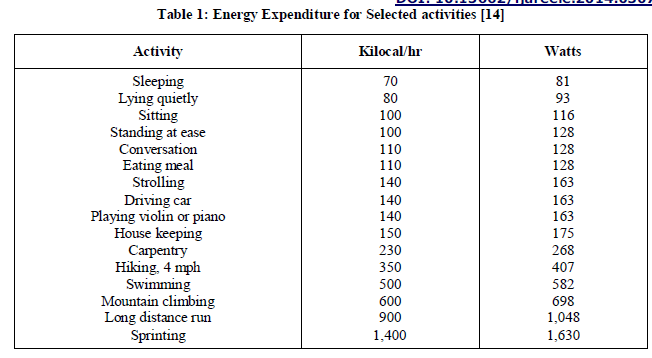 |
d) Power from Typing Keyboard: |
| Keyboard (HandyKey’s Twiddler®) is an important part of computers which plays a vital role in today’s life. As such, it will be used as source of energy. For e.g. Approximate 130 grams of pressure is required to depress a key by 1 mm for entering data. |
| Energy from 1 keystroke = (0.13 kg) X (9.8m /sec^2) X (0.001) =1.3 mJ per key stroke. So, sufficient amount of energy can be drawn from continuous typing [14]. |
| e) Charging from Shoes: Piezoelectric elements are used as pressure transducer which converts pressure energy into electrical energy. Piezoelectric element produces maximum power when we apply specific frequency pressure energy. In order to maximize power multi beam layer of piezoelectric crystal is used [15]. |
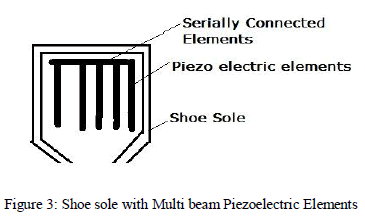 |
| This idea leads us propose to a model using piezoelectric elements to harvest energy for electronics mobile gadget. A sufficient amount of pressure is applied on shoes during walking. So an idea to insert a multi layer piezoelectric elements in shoes to convert this pressure into electrical energy. To achieve a suitable electrical energy, we select piezoelectric elements by different experiments and simulation on software [16]. |
| Power generated by piezoelectric electric element is pulsating in nature so we convert into DC by using AC-DC converter and after that this is fed to storage element or ready for use. Block diagram is given below. |
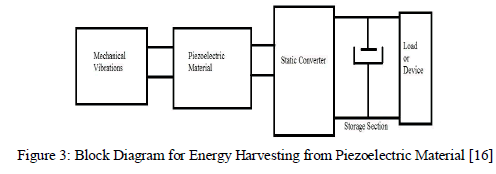 |
| (4) Future Charging Techniques: In the recent past, mobile electronics gadgets charged by wired charger. But now a day, wireless charging accessories going to be popular and next decade will be of wireless charging. |
| Wireless charging Techniques: Maxwell’s equations, which were formulated in 1862, are basically the first theoretical basis of Wireless Power Transmission (WPT). He conceded out the first WPT experiments at the end of 19th century. He tried to broadcast approximately 300 kW power via 150 kHz radio wave. Unfortunately, he failed because of diffusion of the wireless power, which depends on the frequency of operation and the size of the transmitting antenna. After Tesla’s failure, the history of radio-wave development focused on wireless communication and remote sensing rather than WPT. However, the advancement of wireless communication and remote sensing technology helped the development of new WPT techniques [17]. |
| Wireless charging operates in a near field conditions in which coil produces a magnetic field that is picked up by the secondary coil in proximity. |
| Types of Wireless Charging: Wireless Charging is classified into three categories: [18] |
| (a) Radio Charging |
| (b) Inductive Charging |
| (c) Resonance Charging |
| Radio charging will serve low-power devices operating within a 10-meter (30 feet) radius from the transmitter to charge batteries in medical implants, hearing aids, watches and entertainment devices. Radio charging can also activate advanced RFID (radio frequency identification) chips through resonantly enhanced induction. The transmitter sends a low-power radio wave at a frequency of 915MHz (frequency for microwave ovens) and the receiver converts the signal to energy. The radio charging method is closest to a regular radio transmitter; it offers high flexibility but has low power capture and exposes people to electro-smog [19]. |
| RF Based Wireless Charging and Energy Harvesting for Low Power Devices: RF energy is currently broadcasted from billions of radio transmitters around the world, including mobile telephones, handheld radios, mobile base stations, and television/ radio broadcast stations. The ability to harvest RF energy, from environment or dedicated sources, enables wireless charging of low-power devices and this give benefits like product design, usability, and reliability. Battery-based systems can be trickled charged to eliminate battery replacement or extend the operating life of systems using disposable batteries. Battery-free devices can be designed to operate upon demand or when sufficient charge is accumulated. In both cases, these devices can be free of connectors, cables, battery access panels, and have freedom of placement and mobility during charging and usage [19]. |
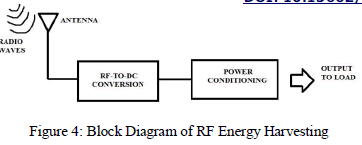 |
| Mobile phones represent a large source of transmitters for harvesting RF energy, and which will potentially enable users to provide power-on-demand for a variety of close range sensing applications. |
| ABI Research, USA and iSupply estimate the number of mobile phone subscriptions has recently surpassed 5 billion, and the ITU (International Telecommunication Union) estimates that over 1 billion subscriptions for mobile broadband exist. |
| Consider the number of Wi-Fi routers and wireless end devices such as tablets, laptops, phablets, smartphones etc. In some urban environments, it is possible to literally detect hundreds of Wi-Fi access points from a single location. At short range, such as within the same room, it is possible to harvest a tiny amount of energy from a typical Wi-Fi router transmitting at a power level of 50 to 100 mW. For longer-range operation, larger antennas with higher gain are needed for practical harvesting of RF energy from mobile base stations and broadcast radio towers. In 2005, Power cast demonstrated ambient RF energy harvesting at 1.5 miles (~2.4 km) from a small, 5 kW AM radio station [18]. |
| b) Inductive charging consist a transmitting and receiving coil in close proximity. Electric toothbrushes were one of the first devices to use this charging method, and mobile phones are the largest growing sector to charge without wires. For mobile charging, simply attach a “skin” that contains the receiver and provides interconnection to the charger socket. Many new devices will have this feature built in [18]. |
| c) Resonance charging basically works on a coil ring which produces an oscillating magnetic field within a radius of one meter (3 feet); the distance between transmit and receive coil must be well within the 1/4th of wavelength (915Mhz has a wavelength of 0.328 meters). Currently, resonance charging in trials can deliver roughly 3,000 watts at a transfer efficiency of 80–90 percent. This is used in larger batteries [21]. |
CONCLUSION |
| In this paper, there is a summary on current batteries technologies researches, discussion about futuristic mobile batteries and how to modify present batteries for their optimize performance by using different anode and cathode materials. It also give light on the introduction of new energy harvesting techniques and proposed new charging techniques which reduce the power loss of conventional charger by step down process.The next generation batteries could provide more energy, last longer & in some cases even cost less than current batteries. Sadly none of them are ready for prime time yet but there is a silver lining and foresee a revolutionary change in Energy Sources for futuristic mobile electronic devices. |
References |
|