ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Mohammad Tajul Islam1, Mohammad Mahbubul Alam2, Marina Zoccola3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Science and technology continue to choose the use of renewable raw materials and more environment friendly and sustainable resources and processes. Nanocellulose is an important addition for that movement. The development of nanocellulose has attracted significant interest in the last few decades due to their unique and potentially useful features. These novel nanocelluloses boost up the strongly expanding field of sustainable materials and nanocomposites. Surface modification of cellulose nafibrils is necessary in order to expand the horizon of their application towards water insoluble polymer matrices. This review organises the current knowledge on the surface modification of microfibrillated and nanofibrillated cellulose from plant sources. A detail of chemical and physical modification of fibril from cellulose has been reviewed.
Keywords |
| Nanocellulose, surface modification, biocomposites |
INTRODUCTION |
| In recent years polymer nanocomposites have been a subject of increasing interest because of their significantly enhanced mechanical properties and thermal stability versus neat polymers or conventional polymer composites [1]. Abundance, high strength and stiffness, low weight and biodegradability are some special useful features of nano-scale cellulose fiber materials which make them promising candidates for bio-nanocomposite production [2]. Isolation, characterization, and search for applications of novel forms of cellulose, variously termed crystallites, nanocrystals, whiskers, nanofibrils, and nanofibers, is generating much activity currently. Isolated cellulosic materials with one dimension in the nanometer range are referred to generically as nanocelluloses. These nanocelluloses are able to combine important cellulose properties such as hydrophilicity, broad chemical-modification capacity, and the formation of versatile semicrystalline fiber morphologies with the specific features of nanoscale materials: features mainly caused by the very large surface area of these materials. Cellulose fibrils with widths in the nanometer range are nature-based materials with unique and potentially useful features. Most importantly, these novel nanocelluloses open up the strongly expanding fields of sustainable materials and nanocomposites, as well as medical and life-science devices, to the natural polymer cellulose. The nanodimensions of the structural elements result in a high surface area and hence the powerful interaction of these celluloses with surrounding species, such as water, organic and polymeric compounds, nanoparticles, and living cells [3]. So far, a certain number of automotive components, appliances and packaging products are now being manufactured using thermoplastic and thermoset natural fibre composites [4]. Application of cellulose nanofibers in polymer reinforcement is a relatively new research field, although there is growing publication activity. The nanofibers are useful only when they demonstrate a reinforcing potential as filler for composite materials. One of the problems encountered in the use of such filler lies in the difficulty in ensuring good dispersion of the filler in the composite material. The phenomenon of agglomeration of the filler is observed in particular with the cellulose fibers used as filler for matrix made of thermoplastic resin. Poor dispersion of the filler in the matrix of a composite material seriously affects its mechanical properties. The limited application of cellulose nanofibers (CNFs) in thermoset and thermoplastic to date may be partly because the dispersion of cellulose nanofibres has typically been a challenging process. More recently, there has been a focus on modification of cellulose nanoparticles, whereby nanofibers are treated by various physical and chemical methods to decrease the energy consumption. Dufresne, Klemm, Siro, Habibi and Azizi Samir have recovered these studies in excellent general reviews on the entire field starting from nanocellulose preparation, modification to application in composites. This review represents specifically various approaches to the surface modification of nanocelluloses. The focus is on the chemical treatments on the surface of nanofibres to make them compatible to petrochemical based thermoset and thermoplastic polymers. |
DISPERSION OF CELLULOSE NANOFIBRES |
| The nanofibers are useful only when they demonstrate a reinforcing potential as filler for composite materials. One of the problems encountered in the use of such filler lies in the difficulty in ensuring good dispersion of the filler in the composite material. The phenomenon of agglomeration of the filler is observed in particular with the cellulose fibers used as filler for matrix made of thermoplastic resin. Nano scale cellulose structures with highly developed specific surface (S) have an increased thermodynamic potential (G), which is the cause of instability of nano objects. According to thermodynamics, to achieve a more stable state, a nano object or phase must decrease its specific surface: |
| ΔG = σ ΔS<0 |
| where σ is the specific surface energy. |
| Therefore, the nano phase has a tendency to form larger structures via aggregation and agglomeration [15]. Poor dispersion of the filler in the matrix of a composite material seriously affects its mechanical properties. CNFs have a high density of –OH groups on the surface, which try to bond with adjacent –OH groups by weak hydrogen bonding. This results in agglomeration or entanglement of the nanofibers; therefore the nanofibers obtained after chemo-mechanical treatments need to keep as water suspension. Mostly water is used as a carrier to disperse cellulose nanofibers. CNFs have a tendency to form hydrogen bonds with adjacent fibrils and hence dispersion is an important challenge in non-polar solvents. The issue of the redispersion of nanofibres after drying is difficult, as irreversible aggregation of the fibrils occurs in a process known as “hornification”, which results in a material with ivory-like properties that can neither be used in rheological applications nor dispersed for composite applications. The main strategy to prevent hornification has been the introduction of a steric barrier or electrostatic groups to block cooperative hydrogen bonding of the cellulose chains [5]. Abundance of hydroxyl groups at the surface of cellulose nanofibres makes it possible to attempt different chemical modifications, including esterification, etherification, oxidation, silylation, polymer grafting, etc. Noncovalent surface modification, including the use of adsorbing surfactants and polymer coating, has been also studied. All chemical functionalizations have been mainly conducted to |
| - introduce stable negative or positive electrostatic charges on the surface of CNFs to obtain better dispersion and |
| - tune the surface energy characteristics of CNFs to improve compatibility, especially when used in conjunction with nonpolar or hydrophobic matrices in nanocomposites. |
| The main challenge for the chemical functionalization of CNFs is to conduct the process in such a way that it only changes the surface of CNFs, while preserving the original morphology to avoid any polymorphic conversion and to maintain the integrity of the crystal [6]. Brief descriptions of some important modification of CNFs are hereinafter summarised. |
| A. Surface modification |
| 1) Acetylation: is beneficial in reducing the moisture absorption of natural fibers. Acetylation of cellulose is one of the best methods for modifying the properties of cellulose fibers. To introduce plasticization to cellulosic fibers, acetylation of natural fibers is a well-known esterification method. Acetylation is originally applied to wood cellulose to stabilize the cell walls against moisture, improving dimensional stability and environmental degradation. Pretreatment of fibers with acetic anhydride substitutes the polymer hydroxyl groups of the cell wall with acetyl groups, modifying the properties of these polymers so that they become hydrophobic. Homogeneous and heterogeneous acetylations of CNFs of Bacterial Nanocellulose (BNC) are possible by using acetic anhydride in acetic acid. In the case of homogeneous acetylation, the partially acetylated molecules immediately partitioned into the acetylating medium as soon as they were sufficiently soluble, while in heterogeneous conditions, the cellulose acetate remained insoluble and surrounded the crystalline core of unreacted cellulose chains. The simultaneous occurrence of cellulose hydrolysis and acetylation of hydroxyl groups has been also reported. Fischer esterification of hydroxyl groups simultaneously with the hydrolysis of amorphous cellulose chains has been introduced as a viable one-pot reaction methodology that allows isolation of acetylated CNFs in a singlestep process [7]. The degree of acetyl substitution had a crucial influence on material properties. Ifuku et al. found that acetylation improved the transparency and reduced the hygroscopicity of BNC/acrylic resin composite materials. However, the composites had an optimum degree of substitution and excessive acetylation reduced their properties. Acetylation has also been reported to improve the thermal degradation resistance of BNC fibers [2]. |
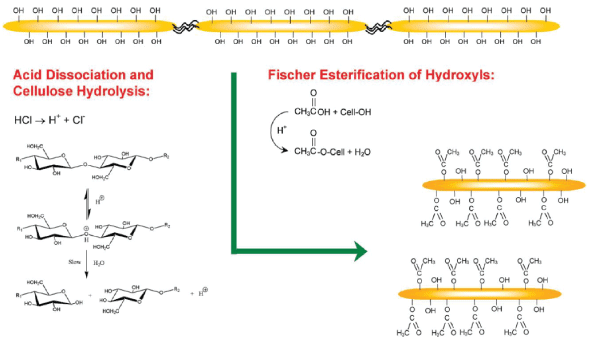 |
| Fig. 1. Reaction scheme illustrating the one-pot (tandem) cellulose hydrolysis and esterification reactivity of hydroxyl groups. Reprinted with permission from Braun, B. et al. [7]. Copyright 2009 American Chemical Society. |
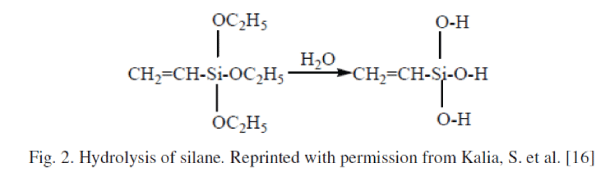
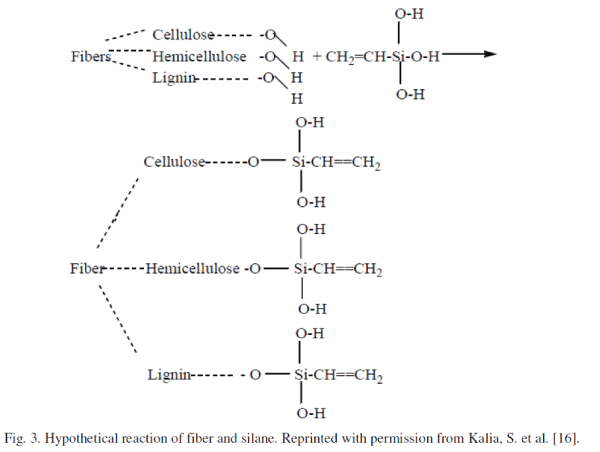
| 2) Silylation: Silane-coupling agents usually improve the degree of cross-linking in the interface region and offer a perfect bonding. Silanes undergo hydrolysis, condensation and bond formation stage. Silanols can form polysiloxane structures by reaction with hydroxyl group of the fibers. The reaction schemes are given in Figure 2 and 3. In the presence of moisture, hydrolyzable alkoxy group leads to the formation of silanols. The silanol then reacts with the hydroxyl group of the fiber, forming stable covalent bonds to the cell wall that are chemisorbed onto the fiber surface. Therefore, the hydrocarbon chains provided by the application of silane restrain the swelling of the fiber by creating a crosslinked network due to covalent bonding between the matrix and the fiber. Cellulose whiskers obtained from the acid hydrolysis of tunicate have been partially silylated by a series of alkyldimethylchlorosilanes, with the carbon backbone of the alkyl moieties ranging from a short carbon length of isopropyl to longer lengths represented by n-butyl, n-octyl, and ndodecyl. It has been demonstrated that with a degree of substitution (DS) between 0.6 and 1, the whiskers became readily dispersible in solvents of low polarity (such as THF) leading to stable suspensions with birefringent behavior, while their morphological integrity was preserved. However, at high silylation (DS greater than 1), the chains in the core of the crystals became silylated, resulting in the disintegration of the crystals and consequently the loss of original morphology [6]. |
 |
 |
| 3) Bacterial cellulose: Lu et al. [9] successfully modified MFC by applying three different coupling agents, 3 aminopropyltriethoxysilane, 3-glycidoxypropyltrimethoxysilane, and a titanate coupling agent [8], with an aim to enhance the adhesion between microfibrils and epoxy resin polymer matrix. The surface modification changed the character of MFC from hydrophilic to hydrophobic while the crystalline structure of the cellulose microfibrils remained intact. Possibly lower polarity of the titanate modifier alkyl chain makes it best among the tested coupling agents to yield the most hydrophobic surface. Unlike silane coupling, titanate coupling is thought to occur via alcoholysis, surface chelation or coordination exchange. When there are hydroxyl groups present on the surface of the substrate, the monoalkoxy- and neoalkoxy-type titanium-derived coupling agents react with the hydroxyl groups to form a monomolecular layer. |
| 4) Grafting: Polymer grafting on the surface of CNFs has been carried out using two main strategies, namely, the “graftingonto” and “grafting-from”. The grafting onto approach involves attachment of presynthesized polymer chains onto hydroxyl groups at the cellulose surface by using a coupling agent. In the “grafting from” approach, the polymer chains are formed by in situ surface-initiated polymerization from immobilized initiators on the substrate [6]. |
| The “grafting onto” approach was used by Ljungberg et al. [18] to graft maleated polypropylene (PPgMA) onto the surface of tunicate-extracted CNFs. The resulting grafted nanocrystals showed very good compatibility and high adhesion when dispersed in atactic polypropylene. The “grafting from” approach applied to CNFs was first reported by Habibi and Vignon [10], who grafted polycaprolactone onto the surface of CNFs via ring-opening polymerization (ROP) using stannous octoate (Sn(Oct)2) as a grafting and polymerization agent. |
| Stenstad et al. reported three methods for modification of MFC by heterogeneous reactions in both water and organic solvents. MFC surface was functionalized with epoxy by oxidation with cerium (IV) followed by grafting with glycidyl methacrylate. Reactive epoxy groups could serve as a starting point for further functionalization with ligands which typically do not react with the surface hydroxyls present in native MFC. A major advantage of this technique is that the reaction is conducted in aqueous media, thereby avoiding the use of organic solvents and laborious solvent exchange procedures. By this method, cellulose nanofibers with a surface layer of moderate hydrophobicity can be prepared. In the same research, a far more hydrophobic MFC surface was obtained by grafting of hexamethylene diisocyanate, followed by reaction with amines. Succinic and maleic acid groups can be introduced directly onto the MFC surface as a monolayer by a reaction between the corresponding anhydrides and the surface hydroxyl groups of the MFC [2]. |
| The potential use of chemically coated hemp nanofibers as reinforcing agents for biocomposites was explored by Wang and Sain et al. [11]. The cellulose nanofibers were treated using five different chemicals: ethylene acrylic acid, styrene maleic anhydride, guanidine hydrochloride, and Kelcoloids® HVF and LVF stabilizers (propylene glycol alginate). Bionanocomposites were prepared from PLA and PHB as matrices. Nanofibers were only partially dispersed in the polymers and therefore mechanical properties were lower than those predicted by theoretical calculations. |
| 5) Surface active agent/ Surfactant: Interaction of cellulose with surfactants has been another way to stabilize cellulose suspensions into non-polar systems. A surfactant is briefly defined as a material that can greatly reduce the surface tension of water when used in very low concentrations. A particular type of molecular structure performs as a surfactant (Figure 4). This molecule is made up of a water soluble (hydrophilic) and a water insoluble (hydrophobic) component. The hydrophobe is usually the equivalent of an 8 to 18 carbon hydrocarbon, and can be aliphatic, aromatic, or a mixture of both. The hydrophilic groups give the primary classification to surfactants, and are anionic, cationic and non-ionic in nature. Noncovalent surface modifications of CNFs are typically made via adsorption of surfactants. This approach has been introduced by Heux et al., who used surfactants consisting of the mono- and di-esters of phosphoric acid bearing alkylphenol tails. The obtained surfactant-coated CNFs dispersed very well in nonpolar solvents. Detailed analyses of the data provided by small angle neutron scattering (SANS) revealed that the surfactant molecules formed a thin layer of about 15 Å at the surface of the CNFs. Use of anionic and non ionic surfactants have been reported [6]. |
 |
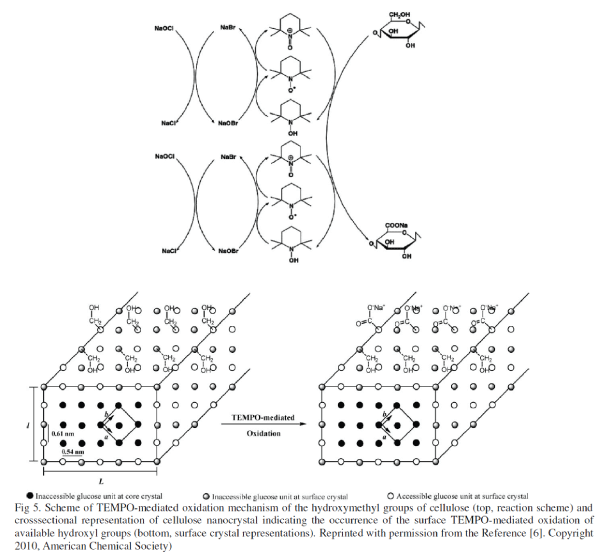 |
| 6) TEMPO Mediated Oxidation: Hydroxylmethyl groups of CNFs present on their surface can be converted to their carboxylic form by oxidation with (2,2,6,6-Tetramethylpiperidine-1-oxyl)-mediated (or TEMPO-mediated). |
| This oxidation reaction, which is highly discriminative of primary hydroxyl groups, is also “green” and simple to implement. It involves the application of a stable nitroxyl radical, the 2,2,6,6- tetramethylpiperidine-1-oxyl (TEMPO), in the presence of NaBr and NaOCl (Figure 5, top). TEMPO-mediated oxidation of CNFs involves a topologically confined reaction sequence, and as a consequence of the 2-fold screw axis of the cellulose chain, only half of the accessible hydroxymethyl groups are available to react, whereas the other half are buried within the crystalline particle (Figure 5, bottom). TEMPO-mediated oxidation of CNFs, obtained from HCl hydrolysis of cellulose fibers, was first reported by Araki et al. [12] as an intermediate step to promote grafting of polymeric chains. These authors demonstrated that after TEMPO mediated oxidation, the CNFs maintained their initial morphological integrity and formed a homogeneous suspension when dispersed in water. The basis for these latter observations was the presence of the newly installed carboxyl groups that imparted negative charges at the CNF surface and thus induced electrostatic stabilization. |
| B. Solvent exchange |
| In addition to chemical modification of nanofibrils, solvent exchange can be a possible route to facilitate dispersion. MFC was premixed with PLA using acetone and the mixture was kneaded after the removal of the solvent to attain uniform dispersion. Resulted composite sheet has significantly more uniform filler distribution compared to sheets prepared via direct introduction of MFC to the molten PLA matrix. As a consequence of uniform distribution the Young’s modulus and tensile strength of PLA increased by 40 and 25% respectively without a reduction of yield strain at a fiber content of 10 wt%. Furthermore, the storage modulus of the composites was kept constant above the glass transition temperature of the matrix polymer [13]. PLA was crystallized when premixed with MFC by using acetone and then exchanged for dichloromethane. Crystallization of PLA increases the storage modulus as well as the strength and Young’s modulus of PLA/MFC nanocomposites without significant reduction in the strain at break [14]. |
| C. Physical modifications of nanofibers |
| Physical treatments change structural and surface properties of the fiber and thereby influence the mechanical bonding with the matrix. Physical methods involve surface fibrillation, electric discharge (corona, cold plasma), ultrasonic, irradiation, electric currents, etc. But only sonification, a simple and versatile approach for dispersion bionanofibers from natural materials using the ultrasonic technique was reported. The cellulose suspension was sonicated with a Branson sonifier [17], behaved as nonflocculating and non-sedimenting aqueous suspensions of cellulose microfibrils. It is made up of individual cellulose fragments consisting of slender rods that have a broad distribution in size. |
CONCLUSION |
| A growing number of research activities have been reported worldwide on the formation and utilization of nanocelluloses over the last era particularly in the last seven years. The use of nanofibres from cellulose has been restricted to polar based polymers due to its lack of compatibility with petrochemical based thermoset and thermoplastic polymers. To expand the horizon of cellulosic nanofibres’ application surface modification is very important. Among all the modification chemical treatments shows more potentiality. Besides the chemical modification some physical treatments recently have been reported like plasma treatment of nanofibrils. |
ACKNOWLEDGEMENT |
| Authors would like to thank the Regione Lombardia for the financial support of the project Ve.Li.Ca (Vegetali Lino Canapa). |
References |
|