Present study is done to establish the hypothesis that alcohol does cause intravascular hemolysis by comparing serum concentration of G6PD in alcoholic patients in comparison to normal healthy control. In this case control & cross sectional study patients were selected from outpatient department of Medicine & Psychiatry, Chirayu Medical College& Hospital, India. Patients between 25—50 yrs of age with a history of drinking alcohol more than 80gms per day for more than 5 years were taken for the study. Serum G6PD concentration is measured by using Method proposed by Kornberg and Horecker (1955) , along with RBC count and indirect bilirubin estimation. It was observed that G6PD activity in serum is in significant amount among the alcoholics. The concentration of serum G6PD increases significantly (3.02 ± 65 IU/ml) along with the decrease in RBC count. Indirect bilirubin (unconjugated bilirubin) was found to be very significantly high (7.536 ±2.28mg/dL) along with decrement in the hemoglobin concentration (12.04 ± 0.60 gm/dl) and RBC count. Serum G6PD shows a negative correlation (r = - 0.70). RBC count and indirect bilirubin was concentration show a moderate negative correlation (r= - 0.55). From the above result we conclude that alcohol does cause intravascular hemolysis and Serum G6PD level can be considered as a marker for intravascular hemolysis.
Keywords |
| Alcoholic liver diseases, serum G6PD, indirect bilirubin, intravascular hemolysis |
Introduction |
| Alcohol causes dysfunction of almost all major organs including brain, liver, heart, pancreas, adrenal gland and thyroid gland. In 1990, the World Health Organization (WHO) estimated that globally alcohol accounts for 3.5% of the total days that are lost due to death and disability [1]. Abusers often continue to consume ethyl alcohol despite knowing that they suffer from alcohol related medical problems along with social and interpersonal problems. |
| Alcohol is a drug which is absorbed from both stomach and small intestine, but is rapidly absorbed from the later. The rate of absorption is variable, alcohol gets distributed throughout the body water, mostly heart tissues, and brain and muscle are exposed to the same concentration as in blood [2]. Ethyl alcohol absorption is very rapid when it is taken in empty stomach. The ethyl alcohol concentration in drink is between 20%- 30%. The concentration of ethyl alcohol in liver is higher which receives blood via the portal vein from stomach and small intestine. It is eliminated predominantly by hepatic metabolism and only 2%-5% is excreted unchanged through urine and breath. |
| Alcohol abuse may result in mild changes in bone marrow and hematological parameters [3].Ethyl Alcohol has several pathological effects on hematopoesis. It directly damages erythroid precursors [4], there by contributing to macrocytosis and the anemic state of chronic alcoholics [5]. Further regular ingestion of alcohol can lead to various types of hemolytic anemia, caused by alteration in the erythrocyte membrane structure which occurs in association with alcoholic liver disease. |
| There are also changes in erythrocyte enzyme activity during erythrocyte aging in alcoholism. Oxidative stress occurs in various forms due to alcohol consumption and alcohol dependence causes gradual exhaustion of the antioxidant capacity of erythrocytes [6]. The activities of Glutathione peroxides and Glucose 6 phosphate dehydrogenase were found to be significantly lower in alcoholics [7]. |
METHODS |
Subject selection |
| Patients were selected from the outpatient department of Medicine & Psychiatry, Chirayu Medical College & Hospital. Each patient was informed about the procedures and consent was taken. Each patient was administered CAG E[8,9] and MAST [10,11] questionnaires and detailed alcohol history were taken for identification of alcohol abusers. 50 alcohol abusers suffering with alcoholic non cirrhotic hepatitis were selected and 50 ages matched normal healthy controls were selected for comparative study. |
Inclusion criteria |
| Control group |
| Normal healthy males aged between 25 – 50 years with no alcohol history included in this group. |
| Experimental group |
| Patients between 25—50 yrs of age with a history of drinking alcohol more than 80gms per day for more than 5 years and not undernourished, were included in this group. |
Exclusion criteria |
| Control group |
| Subjects who are Chronic smokers, having history of moderate alcohol consumption, hypertensive, and or having history of diabetic complications, nephrotic diseases, thyroid disease , pulmonary complications, anemia, hepatitis were excluded from the study. |
| Experimental group |
| Subjects who are undernourished and having history of consuming alcohol for less than five years, chronic smokers, hypertensive, and or having history of diabetic complications, nephritic diseases, thyroid disease, pulmonary complications, were excluded from the study. |
| Venous blood was withdrawn without stasis before commencement of any treatment for the estimation of biochemical and physiological parameters. |
Determination of Serum Glucose 6 Phosphate Dehydrogenase (EC 1.1.1.49) |
| Method proposed by Kornberg a and Horecker[12] (1955) |
| The assay system contained 1ml of 0.02M glucose 6 phosphate, 0.1 ml of 0.0015M NADP, 0.25 ml glycine buffer (0.04M , pH 7.5) and 0.2 ml of 0.1M MgCl2 to this 50μl enzyme source( i.e., serum) was added and change in absorbance, which is due to rate in increase in NADPH concentration, was measured at 340nm at 1minute interval. |
Determination of serum bilirubin |
| Direct and indirect bilirubin was measured in serum with fully automatic analyzer Cobas C111 (Roche Diagnostic, Germany) |
Hemoglobin estimation |
| Cynmethaemoglobin (Drabkin’s) [14] method |
RBC count was done with the automated cell counter. |
Statistical Analysis |
| Data were analyzed using Pearson’s Correlation between different study variables and Z test for the test of significance, p<0.05 was considered as significant. All the test were performed using SPSS Windows version 8.0. Scatter diagram and line diagrams were created to reveal the concept. |
RESULTS |
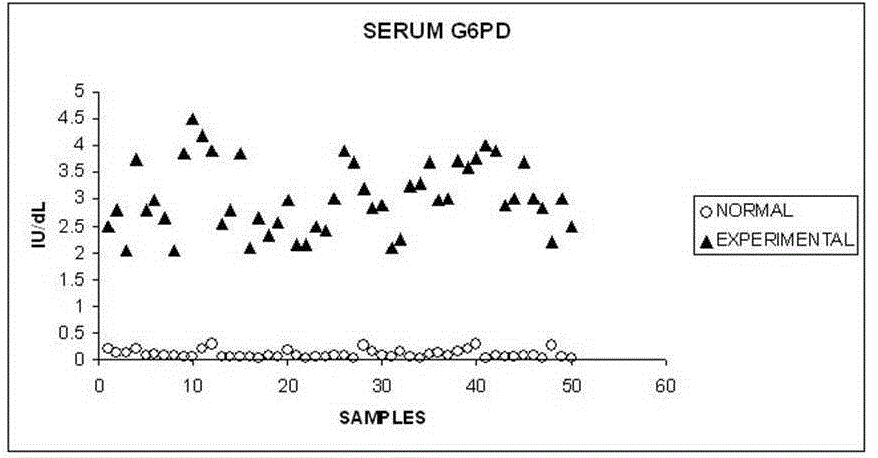
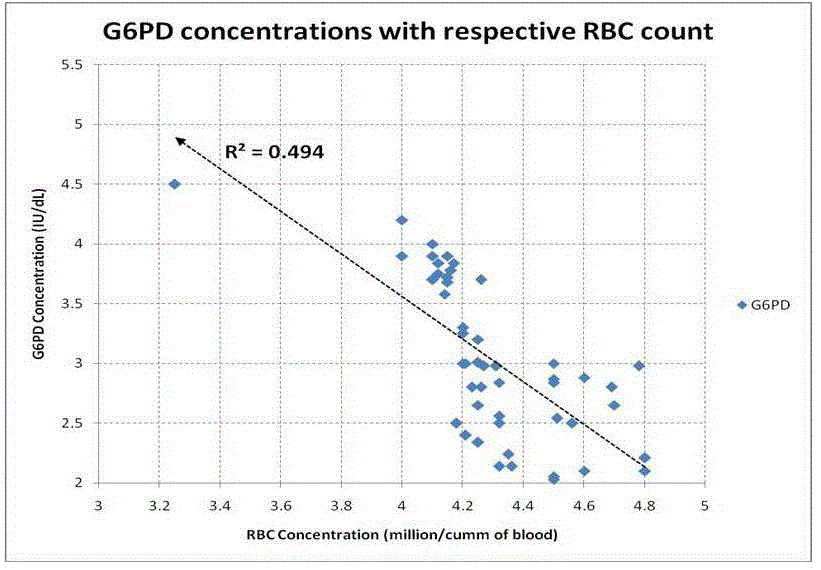
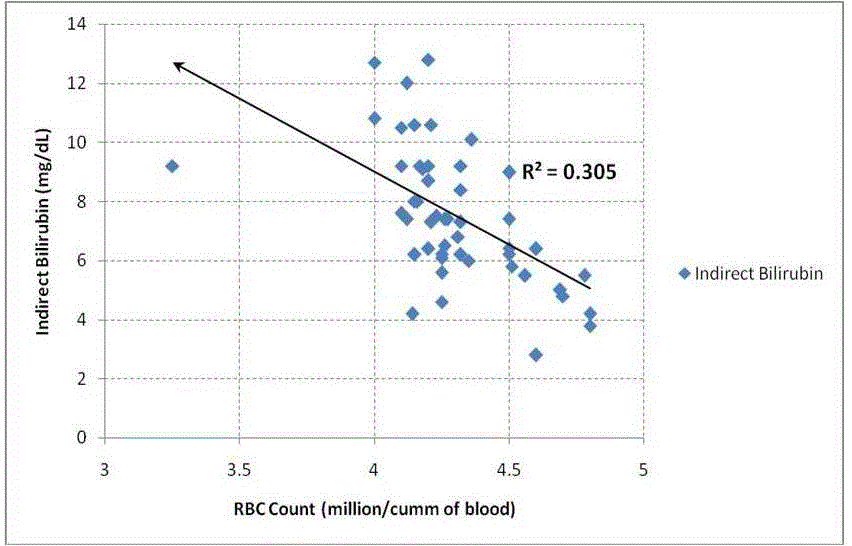
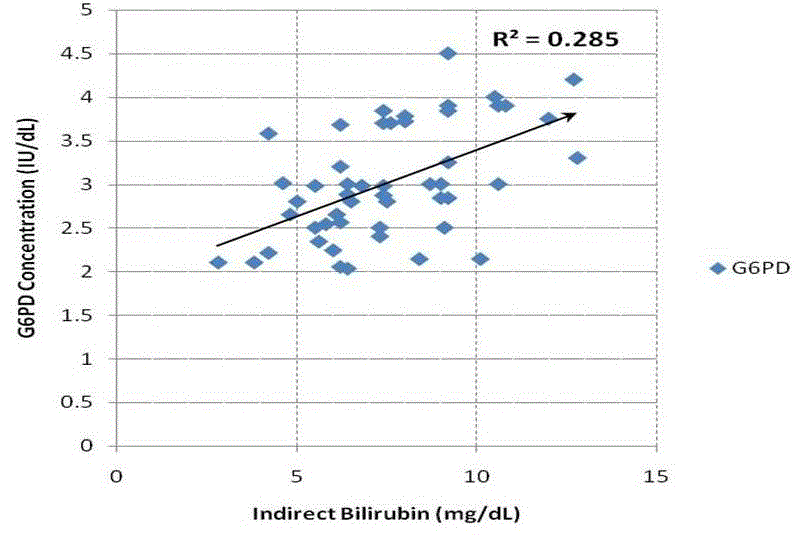
| Table 1 summarizes the outcome of the study. We have found that RBC count decreased significantly {(p<0.05) Table: 1} in alcoholic hepatitis patients. The changes in serum G6PD also increase significantly {(p<0.05) Table: 1}. bilirubin level was found to be increased in alcoholic patients. Indirect bilirubin (unconjugated bilirubin) was found to be very significantly high. It is found that RBC count and serum G6PD shows a strong negative correlation {(r = - 0.70); Table: 2, Fig: 2}. When RBC count and indirect bilirubin was compared they show a moderate negative correlation {(r= - 0.55); Table: 2, Fig: 3}. The Indirect Bilirubin and G6PD concentration lies in moderate positive correlation {(r= 0.53) Table: 2, Fig: 4}. |
DISCUSSION |
| In the present study patients with alcoholic liver disease were compared with a group of normal healthy persons. It has been reported that alcohol has marked effect in hepatobiliary and hematological parameters even in subjects without serious liver damage. [15] Increased oxidative stress has been put forward as one possible mechanism behind alcohol related tissue damage [16]. As alcohol has many loci of action, one deleterious factor in ethanol metabolism is the potential to generate excess amount of free radicals. The extent to which this activity accounts for the overall toxicity of alcohol is unknown. Acetaldehyde catabolism also has the likelihood of contributing to ethanol related oxidative stress [17]. RBCs are subjected to oxidative stress during their normal aerobic functions. Acetaldehyde catabolism also has the likelihood of contributing to ethanol related oxidative stress [17]. RBCs are subjected to oxidative stress during their normal aerobic functions. |
| But in normal healthy subjects this stress is balanced by a powerful enzymatic and non-enzymatic anti-oxidant system failing which may probably disturb capillary perfusion and resulting in cell lysis [18]. It is concluded that the destabilizing effect of unmetabolised ethanol occurs under conditions of acidic hemolysis, whereas destabilizing effect of the oxidation of ethanol to acetaldehyde takes place only under condition of oxidative hemolysis [19]. |
| Alcohol can be directly toxic to the bone marrows suggested by proerythroblastvacuolation or sideroblastic changes. As an indication of hemolysis experiments with intravenous ethanol in mini pigs shows increased indirect bilirubin (unconjugated bilirubin) and total bilirubin along with parallel increase of serum hemoglobin[20] . It has been seen that a proportion of alcoholics have both reduced erythrocyte production in the bone marrow and as well reduced blood cell survival time[3]. |
| G6PD is one of the enzymes that are expressed in all living organisms [21] .This is the first enzyme of the hexose monophosphate shunt pathway and the main intracellular source of reduced nicotinamide adenine di nucleotide phosphate (NADPH) [22].The Glucose 6 phosphate dehydrogenase (E.C. 1.1.1.49; D-glucose-6-phosphate: NADP oxidoreductase) [23]remains in two form within the RBC(dimeric&tetrameric)[24] .G6PD maintaining NADPH in its reduced state, an adequate amount of which is required to regenerate sulfahydryl containing proteins such as Glutathione from oxidized to reduced state. Glutathione in its reduced form in turn protects Hb from oxidation. Hemolysis is experienced when erythrocytes are exposed to oxidizing agents as oxidation of Hb and damage to cell membrane occurs with generation of free radical and lipid peroxidases[25,26]. |
| Formation of Hb adduct with acetaldehyde indicate potential damage to RBC [27]. Alcohol abusers can develop a characteristic sideroblastic anemia, often accompanied by impaired folate metabolism or folate deficiency [3]. Chronic alcohol abuse can lead to various type of hemolytic anemia caused by alteration in the erythrocyte membrane lipids, counting for decrease in the RBC count [28, 26] . |
| G6PD is an intracellular enzyme and barely shows any serum activity [29]. But in our study we have found that serum activity is highly increased in alcoholic liver disease patients (fig 1). |
| Yet there appears to be no definitive evidence that alcohol directly affects circulating erythrocytes in a way that alters their survival [30]. But in our study we found a strong negative correlation between the erythrocyte counts with serum G6PD activity (fig 2). In the present study we found that there is an increase in G6PD values in the serum of alcoholic patients when compared to the normal healthy individuals. The serum values are used for evaluation of enzyme elevation. Our finding of negative correlation between G6PD in serum and RBC count (r= -0.70) is in agreement with the observation that hemolysis resulting due to alcohol consumption may be the cause of presence of the enzyme (G6PD) in the serum. |
| It is known that the concentration of ethanol in bile generally higher than the arterial blood [31]. It is seen that the serum level of deconjugating enzyme, beta glucuronidase is raised in ethanol administration. This could provide another explanation for the increased fraction of unconjugated bilirubin which accompanies chronic ethanol administration [30]. In neonates also it is suggested that hemolysis in G6PD deficiency is only partly responsible, rather hyper bilirubinemia is due to decreased bilirubin conjugation and elimination [32]. In our study we found that both conjugated and unconjugated bilirubin are significantly increased in alcoholic subjects, which is suggestive of no such hepatic greater insufficiency of conjugation if minimally exist. The increase in the serum unconjugated bilirubin is with negative correlation with the RBC(r= -0.55) within the alcoholic group and it is also significant (p<0.05) (fig 3). |
CONCLUSION |
| The main finding of the present study is that 1. Chronic alcohol abusers have significantly high serum G6PD activity. 2. Abusers have shown significant decrease in total number of RBC. |
| From our study it can be concluded that alcohol does cause hemolysis of circulating erythrocytes and there is increment in the serum G6PD value with the degree of hemolysis, so Serum G6PD may also be considered as one of the marker for hemolytic assault in chronic alcoholic liver diseases. Estimation of Seurm G6PD as hemolytic marker is much cost effective than the present available markers for hemolysis. Further studies are required to establish the sensitivity of serum G6PD as hemolytic maker with respect to other available markers. |
References |
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498-504.
- Paton A. ABC of Alcohol,London: BMJ Publishing Group,1994
- Gemma Lewis, Matthew P Wise, Christopher Poynton, Andrew Godkin. A case of persistent anemia and alcohol abuse. Nature ClinPractGastroenterolHepatol. 2007;4:521âÃâ¬Ãâ526.
- Panasuik A, Kemona A. Bone marrow failure and hematological abnormalities in alcoholic liver cirrhosis. RoczAkad Med Bialymst. 2001;46:100âÃâ¬Ãâ105.
- Maruyama S, Hirayama C. Yamamoto S, Koda M, Udagawa A, kadowaki Y, Inoue M, Sagayama A, Umeki K. Red blood cell status in alcoholic and non alcoholic liver disease. J Lab Clin Med. 2001;138(5); 332 âÃâ¬Ãâ 339.
- HagymÃÆási K, BlÃÆázovics A, Lengyel G, Kocsis I, FehÃÆér J. Oxidative damage in alcoholic liver disease. European J GastroenterolHepatol. 2001;13(1):49-53.
- M Maneesh, Sanjiba Dutta, AmitChakrabarti, DM. Vasudevan.Alcohol abuse duration dependent decrease in plasma testosterone and anti oxidants in males. Indian J PhysiolPharmacol. 2006;50 (3): 291âÃâ¬Ãâ296.
- Beullens J. Aertgeerts B. Screening for alcohol abuse and dependence in older people using DSM criteria:A review. Aging and Mental Health. 2004;8(1):76-82.
- JA Ewing. Detecting Alcoholism: The CAGE Questionnaire. J American Med Assoc. 1984;252: 1905-1907.
- Selzer M.L. The Michigan alcoholism Screening test: the quest for a new diagnostic instrument. American J Psychiatr. 1971;127:1653 âÃâ¬Ãâ 1658.
- Hirata ES, Almeida OP, Funari RR, Klein EL. Validity of the Michigan Alcoholism Screening Test (MAST) for the Detection of Alcohol-Related Problems Among Male Geriatric Outpatients. American J GeriatrPsychiatr. 2001;9:30-34.
- Kornberg and Horecker, Determination of glucose-6-phosphate dehydrogenase., Spectrophotometric method. Boehringer leaflet, 1955.
- Malloy HT, Evelyn KA. J Biol Chem. 1955;119-481.
- Cook JD. Measurement of iron status. A report of the International Nutritional AnemiaConsultive Group (INCAG).NewYork : Washington DC;1985, Ch.11: pp 4
- Myrhed M, Berlund L, Bottiger LE. Alcohol consumption and hematology. Acta Med Scand. 1977;202(1-2):11-5.
- Feillet-Coudry C, Rock E, Coudry C, Grezelkowska K, Azais-Braesco V, Drdavent D, et al. Lipid peroxidation and antioxidant status in experimental diabetes. ClinChimActa. 1999;284:31-43.
- Bondy SC. Ethanol toxicity and oxidative stress. ToxicolLett. 1992;63(3):231-241.
- Fernandez Checha JC, Kaplowitz N, Colell A, Gracia-Ruiz C. Oxidative stress and alcoholic liver disease. Alcohol Health Res World. 1997;21:321-324.
- Tyulina OV, Huentelman. Ethanol metabolism affect erythrocyte hemolysisBiochemBiophys Acta.2000;1535(1):69 âÃâ¬Ãâ 77.
- Schwesinger WH, Kurtin WE. Effects of ethanol infusion on serum hemoglobin and bile pigments. J Surg Res. 1984;37:43âÃâ¬Ãâ47.
- Beutler E. Selectivity of proteases as a basis for tissue distribution of enzymes in hereditary deficiencies. ProcNatlAcadSci USA. 1983;80:3767-3768.
- Javier Fernando Bonilla,Magda Carolina Sanchez, LilianChuaire. Glucose âÃâ¬Ãâ 6 âÃâ¬Ãâ Phosphate dehydrogenase (G6PD).Response of the human erythrocyte and another cells to the decrease in their activity. Colomb Med. 2007;38:76âÃâ¬Ãâ83.
- IUPAC. The International Union of Pure and Appliedchemistry.Actualizada 30 de enero de 2007 (fecha de acceso 5 de febrerode 2007). URL disponibleen: http://www.iupac.org/
- Kirkman HN, Hendrickson EM. Glucose 6-phosphate dehydrogenase from human erythrocytes. II. Subactive states of the enzyme from normal persons. J Biol Chem. 1962;237:2371-2376.
- AreseP, and Flora A. Pathophysiology of Hemolysis in G6PD deficiency. Seminars in Hematology: 1990;27:1-
- A Geetha, M. D Lakshmi Priya, S Annie Jeyachristy& R Surendran. Level of oxidative stress in the red blood cells of patients with liver cirrhosis. Indian J Med Res. 2007;126;204-210.
- R Britton, B Bacon. Hereditary hemochromatosis and alcohol: A fibrogenic cocktail. Gastroenterol. 2002;122(2):563 âÃâ¬Ãâ 565.
- Nordman R, Ribiere C, Rouch H. Ethanol-induced lipid peroxidation and oxidative stress in extrahepatic tissues. Alcohol. 1990;25:231-237.
- VarleyâÃâ¬Ãâ¢s Practical Clinical Biochemistry. 6th Edition, Heinemann Professional Publishing Ltd. London.
- Wayne H Schwesinger, William E Kurtin, Barry A. Levine, Carey P Page. Cirrhosis and Alcoholism as Pathogenic Factors in Pigemtn Gallstone Formation. Ann Surg. 1985;319âÃâ¬Ãâ322.
- Beck IT, Paloschi GB, Dinda PK, et al. Effect of intragastric administration of alcohol on the ethanol concentrations and osmolality of pancreatic juice, bile, and portal and peripheralblood. Gastroenterol. 1974;67:484-489.
- S Behjati- Ardakani, ANikkhah, M. Sedaghat. Association between G6PD deficiency and total serum bilirubin level in icteric neonates ActaMedicaIranica. 2007;45(3):233 âÃâ¬Ãâ 235.
|