e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry and Biochemistry, Stephen F Austin State University, Nacogdoches, Texas, USA
Received date: 10/11/2015; Accepted date: 27/11/2015; Published date: 30/11/2015
Visit for more related articles at Research & Reviews: Journal of Chemistry
Among the heavy metals, mercury is often considered the most toxic; moreover, current methods used to sense and quantitate mercury in the environment are expensive and labor intensive. In response, the sensor community has sought new approaches to detect and quantitate mercury. One such approach is the use of porphyrins as selective reagents for the detection of mercury. Porphyrins have shown great promise in sensing applications because of their intense absorptions in the visible region, fluorescence properties, rich redox chemistry and most importantly selective, reversible association with mercury ions. In this review, we presented some of the most remarkable developments with porphyrin based solid state sensors over the past ten years (2006-2015) for the recognition and sensing of mercury (II) ions in aqueous solution. Attention is focused on the synthetic route, the immobilization scheme used to associate the porphyrin with a solid support, the spectroscopic method used to sense mercury ions, and the limit of detection for the sensor
Porphyrin, Mercury ion sensors, Heavy metals, Analytical techniques, Spectrometry, X-ray fluorescence, Ion chromatography, UV/vis, Toxic Metals.
In recent years, the scientific community has shown interest in developing new tools for the detection and quantitation of biologically and environmentally relevant metal ions. Currently, expensive analytical techniques, such as, atomic absorption spectrometry, X-ray fluorescence, ion chromatography, and inductively coupled plasma mass spectrometry are being used to detect and quantitate metal ions [1]. These techniques require dedicated laboratory space, extensive sample preparation, and highly trained personnel. Thus, finding an alternative approach has become an important topic of research among the sensor community. Porphyrins based probes have shown great promise because of a number of reasons. First, they exhibit characteristic electronic absorptions in the Soret band region (~400 nm, S0→S2) as well as in the Q-bands region (500-700 nm, S0→S1) [2,3]. Second, the porphyrin core is planar and rigid leading to fluorescence. Third, porphyrins have rich redox chemistry. Most importantly, porphyrins are sensitive to metal ions, typically form a sitting-atop chelation complex with various heavy metal ions. After complexation with heavy metal, metalloporphyrins retain its photophysical properties. Metalloporphyrins exhibit a strong intense, ε ≈105 M-1 cm-1, absorption band in the visible region (380 to 420 nm) [2,3]. Metalloporphyrins also exhibit a strong fluorescence quenching (500 to 700 nm, S1→S0) with heavy metal ions [4-13]. Metalloporphyrins have found applications in potentiometery, voltammetry and as electrochemical biosensors [14]. Despite its excellent photophysical properties, these molecules possess very poor water solubility. This along with other reasons (see in section 2) prompted the scientific community to develop porphyrin solid state sensors for detecting and sensing heavy metal ions in biological and environmental aqueous solution.
Among the toxic heavy metals, mercury is considered as the most harmful environment pollutant. The United Nations Environment Programme (UNEP) estimated that the anthropogenic emission of mercury in 2010 was 1960 tonnes [15]. Mercury has 5 major forms: elemental mercury liquid, elemental mercury vapor, the mercury (II) ion also known as the mercuric ion, the mercury (I) ion also known as the mercurous ion and organic mercury ions such as methyl mercury. All forms of mercury are toxic and have important implication to human health [16]. The mercury (II) salts are, in general, more toxic than metallic mercury [16,17]. Mercury permeates the skin, respiratory and gastrointestinal tissues easily [16]. Once in the body, it disrupts the biological activity in the cells by binding with proteins and nucleic acids [16,17]. Small concentrations of mercury metal ions have a detrimental impact on the healthy development of children, by seriously damaging the central nervous system [16,17].
In this review, we presented some of the most remarkable developments in porphyrin based solid state sensors over the past ten years (2006-2015) for the recognition and sensing of mercury (II) ions in solution, particularly, in aqueous solution. This review is not comprehensive; attention is focused on (i) efficient synthetic route used to generate porphyrin sensing reagents, (ii) various approaches used to immobilize the sensing reagent (porphyrin) onto a solid support, (iii) the different analytical and qualitative methods for sensing mercury ions, and (iv) the limit of detection (LOD), and dynamic and linear range of mercury ions detection in aqueous medium.
As mentioned previously, porphyrins are good fluorimetric materials. This remarkable property has become an important tool to the sensor community for rapid sensing of metal ions with high efficiency and high selectivity. However, the poor water solubility, low fluorescence quantum yields in aqueous medium and slow rate of heavy metal complex formation put constraints for its wide application for sensing metal ions in aqueous medium. In response to these problems, various approaches have been taken, one such attempt is solid-state sensor in which commercially available porphyrins or laboratory synthesized porphyrin derivatives are anchored with a solid support by strong covalent bonds or electrostatic attraction between two oppositely charged species. This review reports some excellent solid-state mercury sensors developed over past ten years.
Amphiphilic Porphyrin 1 on Glass
In 2006, a solid-state fluorescence sensor was prepared using an amphiphilic porphyrin 1 (Figure 1) electrostatically held onto a glass substrate for sensing Hg2+ ions in aqueous solution [9]. The porphyrin 1 was synthesized by reacting (3-bromopropyl)- trimethyl-ammonium bromide with free phenolic porphyrin via Williamson coupling [18,19]. The intermediate bromide salt was converted into chloride salt by reacting it with excess sodium chloride (NaCl). The solid-state sensor was prepared by immersing glass slides (solid support) into the aqueous solution of the porphyrins. The charged porphyrin molecules strongly adsorbed onto the glass surface through electrostatic attraction. The solid sensors were robust and have shown an appreciable fluorescence emission. A sharp decrease of fluorescence intensity was found when the sensor was immersed into aqueous solution of mercury ions. The limit of detection LOD of Hg2+ was 1 μM. A linear range for fluorescence quenching was observed for amphiphilic porphyrin on glass sensor from 1.0 × 10-6 M — 2.5 × 10-4 M, using an excitation wavelength of 420 nm and an emission wavelength of 654 nm. The fluorescence lifetime for the solid state sensor was 0.5 ns. The sensor showed an excellent reproducibility; a complete recovery of the fluorescence intensity was observed when the mercury ion saturated solid sensor was rinsed with the chelating agent (N,N,N’,N’-tetrakis(2-pyridilmethyl)ethelenediammine). The sensors have shown a great selectivity toward mercury ions as evidenced by its unperturbed fluorescence intensity in the presence of other heavy metal ions (Cd2+, Pb2+, Zn2+ and Cu2+).
Cationic Porphyrin on Glass
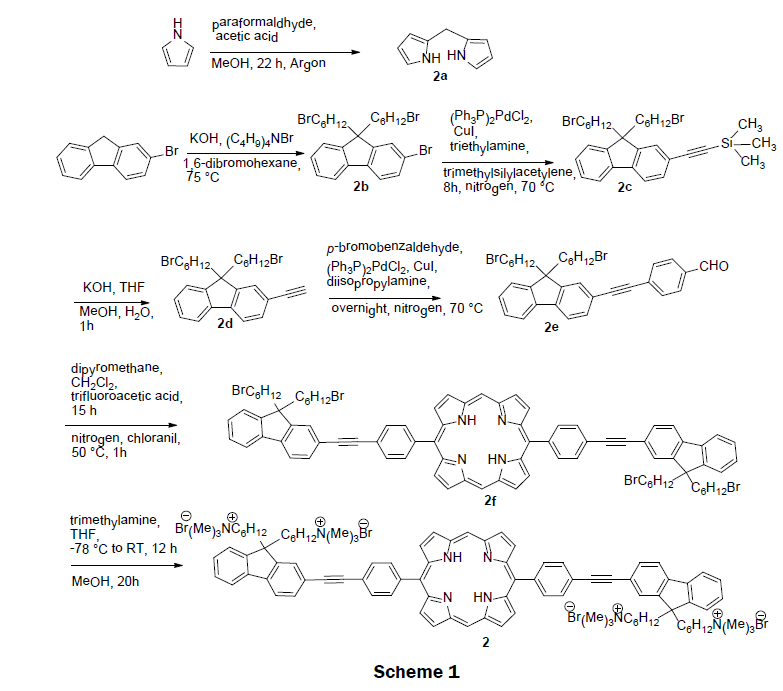
In 2008, a self-assembled solid-state sensor was synthesized using the cationic porphyrin (5,15-(p-(9,9-bis(6- trimethylammoniumhexyl)-fluorenylethynyl)phenyl) porphyrin tetrabromide) 2 and a glass solid support [10]. The preparation of cationic porphyrin 2 involves a tedious multistep organic synthesis. Scheme 1 depicts the synthetic route for 2. First, two precursors, dipyrromethane (2a) and 2-bromo-9,9-bis(6-bromohexyl) fluorene (2b) were prepared according to reported procedure [20,21]. 2-(9,9-bis(6-bromohexyl)fluoren-7-yl) ethynyltrimethylsilane (2c) was obtained from a Sonogashira coupling reaction of 2-bromo- 9,9-bis(6-bromohexyl) fluorene (2b) with trimethylsilylacetylene. After deprotection of trimethylsilane, 9,9-bis(6-bromohexyl)-2- ethynyl-9H-fluorene (2d) was reacted with p-bromobezaldehyde to form p-(2-(9,9-bis(6-bromohexyl)-9H-fluoren-7-yl)ethynyl)- benzaldehyde (2e) in a yield of 51%. A modified MacDonald condensation reaction of p-(2-(9,9-bis(6-bromohexyl)-9H-fluoren-7-yl) ethynyl)-benzaldehyde (2e) and dipyrromethane (2a) afforded neutral conjugated porphyrin derivative (2f). The final 5,15-(p-(9,9- bis(6-trimethylammoniumhexyl)-fluorenylethynyl)phenyl) porphyrin tetrabromide 2 was obtained by reacting neutral conjugated porphyrin derivative with trimethylamine in 65% yield. The pure cationic porphyrin 2 was then immobilized on glass materials to prepare solid sensor. Glass microscope slides were activated with a mixture of concentrated NH4OH and 30% H2O2 for several minutes, rinsed with Millipore water and dried. The slides were then rendered hydrophobic by immersion in hexamethyldisiloxane. The sensor was prepared by incubating the hydrophobic slides into micromolar solutions of the porphyrin complex 2. Layers of porphyrin were controlled by adjusting the hydrophobicity of the slides or the concentration of the porphyrin 2 incubation solution. The cationic porphyrin 2 on the glass has good mechanical strength. Addition of mercury ions quenches the fluorescence emission of 2. The dynamic range of the sensor was 1 × 10-6 M to 1 × 10-10 M. An impressive detection limit was estimated to be 1 × 10-10 M. The electronic absorption and fluorescence spectra of metal-free sensor 2 remain almost unchanged upon addition of other metal ions, such as Cu2+, Zn2+, Pb2+, Cd2+, Mn2+, Ni2+, Co2+, and Ca2+. The quantum yield of the cationic porphyrin 2 was measured ~10% in methanol, while 5,10,15,20-tetra(p-sulfonatophenyl)porphyrin (TPPS) has ~12% in water.
A Porphyrin-Containing Conjugated Polymer Electrolyte on Glass
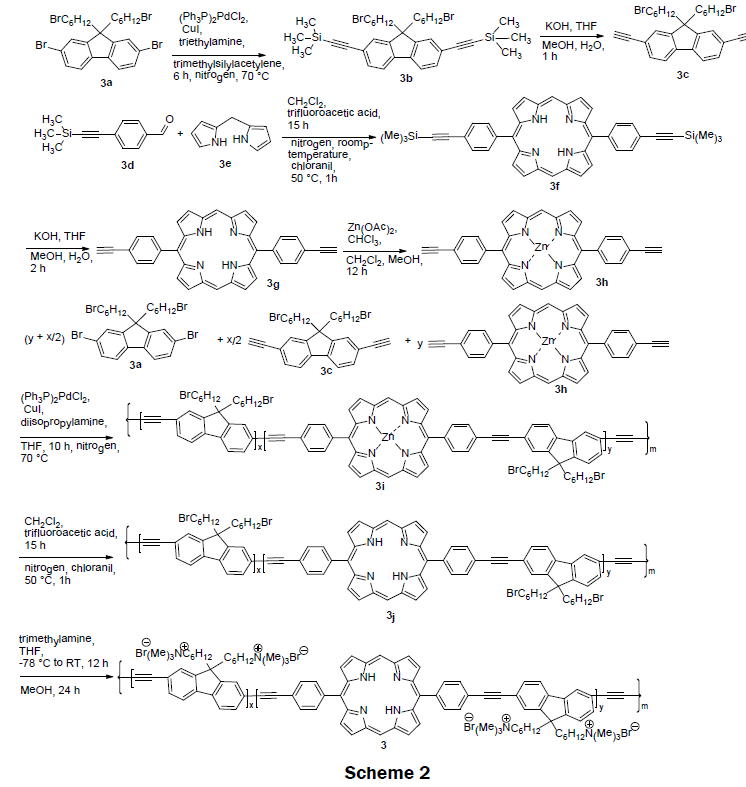
In 2008 [4], a series of cationic dual-emissive porphyrin-containing conjugated polymer electrolyte were synthesized (Scheme 2). These polymer electrolytes were prepared via a multistep processes. Scheme 2 shows the synthetic route for 3. A Sonogashira coupling reaction of 2,7-dibromo-9,9’-bis(6-bromohexyl)fluorene (3a) and trimethylsilylacetylene was conducted to obtain 2,7-ditrimethylsilylethynyl- 9,9’-bis(6-bromohexyl) fluorene (3b) in 75% yield. Deprotection of trimethylsilyl groups afforded the first monomer, 2,7-diethynyl-9,9’-bis(6- bromohexyl)fluorene (3c) in 90% yield. MacDonald condensation of dipyromethane (3e) and 4-(2-(trimethylsilyl)ethynylbenzaldehyde (3d) followed by recrystallization from chloroform/hexane afforded 5,15-p-trimethylsilylethynylphenyl porphyrin (3f) in 41% yield. After removal of trimethylsilyl group, 5,15-(p-ethylphenyl)porphyrin (3g) was obtained in 86% yield. The second monomer, 5,15-(p-ethylphenyl) zincporphyrin (3h) was synthesized from 5,15-(p-ethylphenyl)porphyrin (3g) and zinc acetate via a metalation reaction. The neutral precursor polymers 3i were prepared by a copolymerization reaction of 2,7-diethynyl-9,9’-bis(6-bromohexyl) fluorene (3c), 5,15-(p-ethylphenyl)zincporphyrin (3h), and 2,7-dibromo-9,9’-bis(6-bromohexyl) fluorene (3a). The polymeric solution contains varying feed ratios of the monomers of 3a and 3c along with 0, 1, 5, and 10 mol% of zincporphyrin (3h), respectively. Zinc metal was removed from the obtained polymer by treating it with trifluoroacetic acid to afford neutral precursor polymers 3j in ~80-90% yield. The cationic conjugated polymers electrolytes 3 were prepared in higher than 95% yield by reacting neutral precursor polymer 3j with trimethylamine. Interestingly, when a non-zinc chelated porphyrin (3g) was copolymerized with the 2,7-diethynyl-9,9-bis(6-bromohexyl) fluorene (3c) the reaction failed; a red insoluble aggregation of the porphyrin was produced instead. Both polyfluoreneethynylene (PFE) and the porphyrin fluoresce at λex=375 nm, λem=~ 430 nm, and λex=375, λem=425 nm, respectively. The porphyrin’s fluorescence is quenched in the presence of mercury ions while the PFE’s fluorescence is quenched with increasing ionic strength of test solution. The LOD was reported as 0.1 μM and the linear range was reported as 0 μM–100 μM. Impressively the naked eye-detection limit for 3 on glass was 10 μM. The quantum yield for the various PFE-porphyrin polymers was reported to be between 13% to 27%. Normally, porphyrins have a quantum yield of less than 10%. The increase of quantum yield is attributed to PFE’s energy transfer to the porphyrin.
A Lipophilic Porphyrin on Poly(vinyl chloride) (PVC) Membrane
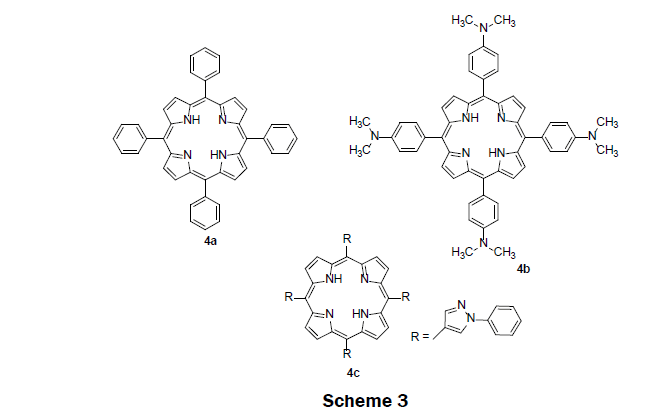
In 2009, three previously synthesized lipophilic porphyrin compounds; tetra (p-dimethylaminophenyl)porphyrin (TDMAPP) 4b, 5,10,15,20-tetraphenylporphyrin (TPP) 4a, and tetra(N-phenylpyrazole) porphyrin (TPPP) 4c were used as sensing reagents in PVC membrane, (Scheme 3) [13]. TDMAPP 4b was found to show higher selectivity and higher sensitivity for Hg2+ ions. The electron donating dimethylamino group on the phenyl helps stronger coordination of TDMAPP with Hg2+ ions. As a result, TDMAPP quenches its fluorescence intensity more effectively than TPP 4a and TPPP 4c. The solid sensor membrane was then prepared by injecting a solution of poly(vinyl chloride), di-iso-octyl sebacate, and TDMAPP dissolve in tetrahydrofuran solution into the center of a rotating quartz slide. The membrane showed high performance for sensing Hg2+ ions. The linear range was observed from 4.0 × 10-8 M to 4.0 × 10-6 M while the LOD was determined to be 8.0 × 10-9 M. The fluorescence of the sensing membrane increased with pH until a pH of 8 where it plateaued. The sensing membrane does not suffer from the interferences including alkali, alkaline earth, and heavy metals. Another appealing feature of the sensing membrane is that it effectively removes Hg2+ ions from contaminated water samples with satisfactory recovery.
Magnetic Sensor Material, Fe3O4@SiO2@Porphyrin
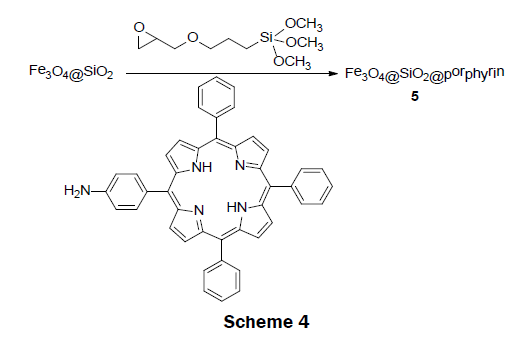
In 2011, an interesting magnetic sensor material, Fe3O4@SiO2@porphyrin 5 was reported [12]. This sensor was easily synthesized with the use of only low-cost materials (Scheme 4). The synthesis was carried out in a step wise process. Fe3O4@SiO2 magnetic material was first prepared according to the reported sol-gel process [22]. The amine porphyrin (TPP-NH2) was synthesized separately by reacting tetraphenylporphyrin (TPP) with fuming nitric acid followed by reduction with tin(II) chloride dihydrate in the presence of hydrochloric acid [23]. The obtained porphyrin-functionalized Fe3O4@SiO2@porphyrin 5 was prepared by anchoring TPPNH 2 to the Fe3O4@SiO2 magnetic material and 3-glycidoxypropyltrimethoxysilane composite (GLYMO-Fe3O4@SiO2) under refluxing conditions. GLYMO-Fe3O4@SiO2 was obtained by a reaction of Fe3O4@SiO2 with glycidoxypropyltrimethoxysilane.. Upon addition of mercury ions, the intensity of emission band at 650 nm and 710 nm gradually decreases and remained unaffected by the presence of other metals ion, such as, K+, Na+, Ba2+, Mn2+, Ca2+, Co2+, Cu2+, Ag+, Ni2+, and Pb2+. In addition, the material showed a visible color change from red to green upon addition of Hg2+ ions, binding the Hg2+ ions reversibly. The LOD and dynamic range was not reported; however, in a subsequent publication it was noted that no color change was seen at a Hg2+ concentration lower than 4 × 10-5 M [24]. The sensor is interesting because it can effectively remove Hg2+ ions from solution by incubating the porphyrin magnetic microsphere in a solution containing Hg2+ and then removing the mercury bound to the magnetic microsphere using an external magnetic field.
Amphiphilic Porphyrin 6 Nanoparticle
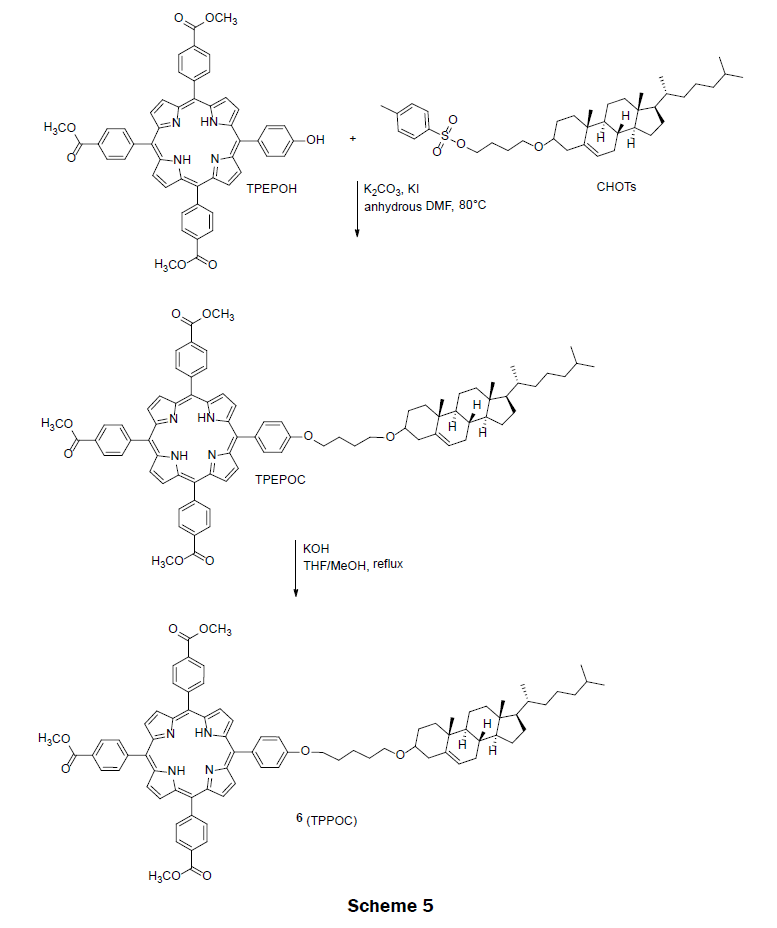
In 2011, an amphiphilic porphyrin, tri-(p-carboxyphenyl)porphyrin-cholesterol dyad (TPPOC) 6 assembly was prepared for detecting organic mercury in water [25]. Organic mercury compounds are not same as inorganic forms of mercury. Typically, inorganic forms of mercury (Hg2+) converts into the organic mercury (RHg(II)+ where R=methyl, ethyl and phenyl) forms by marine organisms. Organic mercury then enters the food chains and accumulates in human bodies leading to human diseases. Organic mercury compounds are considered more detrimental compare to inorganic form of mercury. Thus, the design and synthesis of amphiphilic porphyrin to detect and analyze organic mercury is of great importance. The amphiphilic porphyrin 6 was prepared from tri-(p-carboxyphenyl)-p-hydroxyphenyl-porphyrin (TPEPOH) and cholesterol tosylate (CHOTs) precursors (Scheme 5). These two precursors were synthesized according to the literatures [26-28]. The intermediate ester TPEPOC was obtained via a nucleophilic substitution reaction of TPEPOH and CHOTs in the presence of a base, potassium carbonate. After basic hydrolysis of ester TPEPOC, the final pure amphiphilic porphyrin (TPPOC) 6 was obtained in 80% yield. TPPOC spontaneously forms nanoparticles of approximately 10 nm in diameter in water with the tri(p-carboxyphenyl) porphyrin portion exposed to solution and the cholesterol portion forming a micelle like structure. The dynamic range of porphyrin 6 on glass for PhHg(II)+ is 0.5 μM to 180 μM with two distinct linear ranges, one from 0.5 μM to 50 μM and the other from 50 μM to 180 μM. The LOD for PhHg(II)+ was reported as 0.67 μM. The nanoparticles not only showed a high performance for sensing organic mercury but also for inorganic form of Hg2+ ions. The nanoparticle changed color from a light pink to an olive yellow in the presences of Hg2+ ions. Monoarmoatic pollutants, alkaline earth metal ions (Ba2+, Ca2+ and Mg2+), and transition metal ions (Pb2+, Cd2+, Co2+, Cu2+, Zn2+, Ni2+, Fe2+ and Ag+) did not interfere with the measurement of PhHg(II)+. Minimal interferences to the measurement of PhHg(II)+ were observed in the presence of Mn2+ and Hg2+ ions.
Benzoporphyrin And Porphyrin-2-yl-Pyridines in PMMA
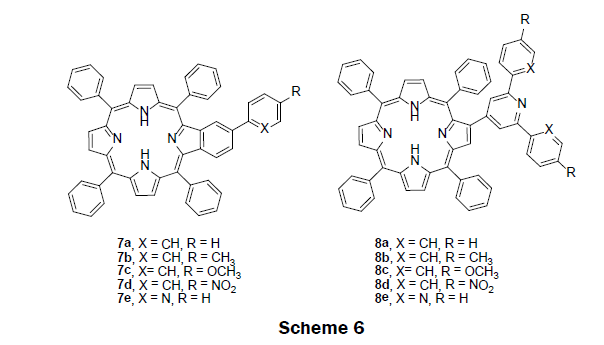
In 2013, the sensing ability of benzoporphyrin 7a-e and porphyrin-2-yl-pyridines 8a-e were investigated in solution prior to the construction of a solid-state sensor (Scheme 6) [29]. A simple and efficient synthetic route, an aldol type condensation and a Kröhnke type reaction was adopted to prepare 7a-e and 8a-e [30]. To examine the compound’s sensing ability, non-aqueous titrations were performed by monitoring both UV/vis and fluorescence spectroscopy of the compounds. Chloroform solutions of the compounds were titrated with Hg2+ ions in acetonitrile solution. The UV/vis spectra of the benzoporphyrin 7a-e exhibited a red shift in the Soret band from 427 nm to 457 nm with the addition of mercury ions; a well-defined isosbestic point was observed at 439 nm suggesting the presence of two species in solution—a free benzoporphyrin species and mercury bound benzoporphyrin species. Likewise, the UV/vis spectra of the porphyrin-2-yl-pyridine 8a-e also exhibited a red shift in the Soret band from 424 nm to 446 nm with the addition of mercury ions; moreover, a well-defined isosbestic point was observed at 420 nm suggesting the presence of two species in solution—a free porphyrin-2-yl-pyridine species and mercury bound porphyrin-2-yl-pyridine species. The fluorescence intensity of the benzoporphyrin compounds 7a-e decreased at 658 nm and 718 nm while a new fluorescence peak appears at 682 nm upon addition of Hg2+ ions. The porphyrin-2-yl-pyridines 8a-e showed typical fluorescence quenching with the addition of Hg2+ ions. Based upon the screening experiments, the benzoporphryin 7a was selected as the best candidate for a mercury ion sensor in solution. The LOD was found to be 0.16 μM Hg2+. The benzoporphyrin 7a was then immobilized into poly(methyl methacrylate) (PMMA) polymer to construct a solid-phase sensor. The preparation of PMMA films was carried out by adding 7a to the PMMA chloroform solution, followed by solvent evaporation under vacuum. Interestingly benzoporphyrin 7a in PMMA film showed similar photophysical properties. A decrease in fluorescence intensity was observed upon addition of mercury ions.
Cross-Linked Copolymer of Tetraphenylporphyrin and Cyclotriphosphazene (TPP-PZS)
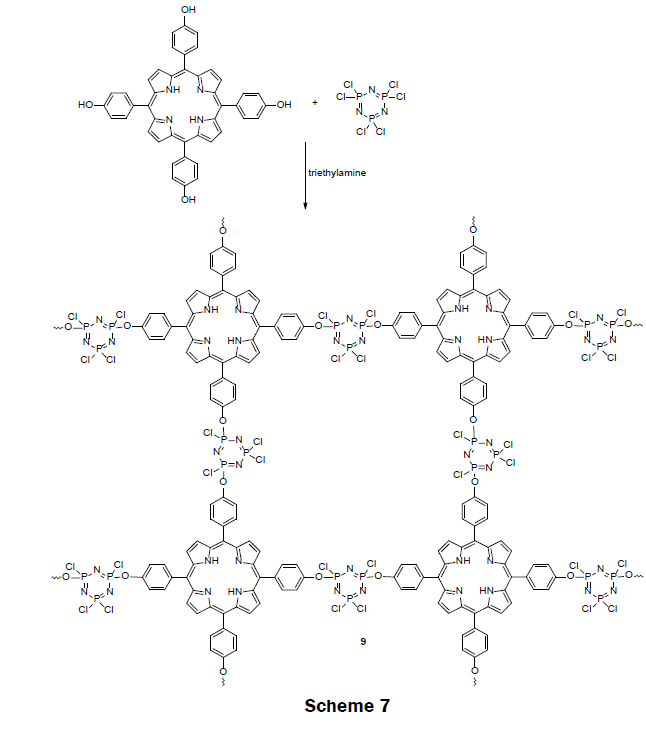
In 2014, a highly cross-linked copolymer of tetraphenylporphyrin and cyclotriphosphazene (TPP-PZS) (9) was prepared (Scheme 7) [5]. In the highly cross-linked network, each porphyrin ring is separated from each other by an insulator cyclotriphosphazene spacer groups. Because of the insulation, porphyrin molecules fluoresce effectively, and are free from concentration—quenching effect. The cross-linked polymer was synthesized by a nucleophilic substitution reaction of hexachlorocyclotriphosphazene (HCCP) and 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin (TPP-(OH)4) in presence of triethylamine (TEA) in acetone or acetonitrile solvent under ultrasonic conditions. The activated hydroxyl group in TPP(OH)4 substituted one chlorine atom from each phosphorus atom in hexachlorocyclotriphosphazene (HCCP). The obtained solid cross-linked polymers were precipitated and separated out from the organic solution, and dried for use. The acetonitrile organic solvent yielded spherical hollow nanoparticles with a narrow size distribution of 250 nm in diameter while the acetone yielded solid, slightly oval nanoparticles having a diameter of approximately 670 nm. Ethanol suspensions (10 mg L-1) of both the hollow and solid nanoparticles were screened for Hg2+, Na+, Ag+, Li+, Ca2+, Mg2+, Ni2+, Co2+, Pb2+, Zn2+, Cd2+, Mn2+, Cu2+, and Fe3+ sensing by exposing the suspension to 10 μM of each metal ion solution. Only the solid nanoparticles, which were polymerized in acetone, exhibited a strong quenching (excitation at 365 nm) at 657 nM for mercury ions. A complete fluorescence quenching of the solid TPP-PZS nanoparticles which were dissolved in ethanol at 10 mg L-1 was achieved with a 8 μM Hg2+ solution. Thereafter, test strips were prepared by immersing ashless filter papers into TPPPZS alcohol solutions. When the test strips were illuminated with 365 nm light, a color change was apparent to the naked eye. A color key for mercury concentrations from 10 μM to 100 μM was reported with the test strips. The LOD and dynamic range for the nanoparticle suspension and the test strips may be adjusted by adjusting the concentration for the suspension of nanoparticles. When the concentration of TPP-PZS suspension was decreased to 0.1 mg L-1 the LOD for Hg2+ is 50 nM. This sensor is a promising one because of its portability and ability to detect and quantitate Hg (II) ion with naked eye.
α,β-Unsaturated Ketone Containing Porphyrin on PMMA
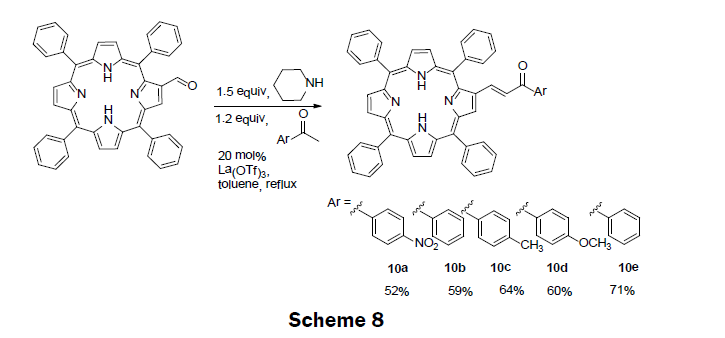
Also in 2014, five porphyrin compounds with an α,β-unsaturated ketones unit (10a-e) were prepared (Scheme 8) [11]. The precursor porphyrin (2-formyl-5,10,15,20-tetraphenylporphyrin) was prepared using standard method by reacting 5,10,15,20-tetraphenylporphyrinate copper (II), with phosphorous oxychloride (POCl3) in N,N-dimethylformamide (DMF) solution [31]. The α,β-unsaturated ketones containing porphyrins were then synthesized by reacting and refluxing an appropriate methyl aryl ketone with 2-formyl-5,10,15,20-tetraphenylporphyrin in presence of piperidine and La(OTf)3 catalyst. The porphyrin compounds are then separated and purified by column chromatography. The % yields obtained for 10a-e are 52%, 59%, 64%, 60%, 71%, respectively. Five derivatives of porphyrin conjugates were screened using spectrophotometric and spectrofluorimetric titrations towards the metal ions Ag+, Cu2+, Zn2+, Cd2+, and Hg2+. Based upon these Results, compound 10d was chosen as a representative example of the derivatives and incorporated into a polymethylmethacrylate (PMMA) polymer. When the polymer-porphyrin 10d was incubated in Hg2+ solution (1 × 10-3 M) the polymer changed color from dark brown to light brown, and an enhancement in the fluorescence was also observed. The LOD and dynamic range for the solid state sensor were not reported; however, non-aqueous titrations of the porphyrin derivatives with mercury ions monitored using UV/vis spectroscopy gave the LOD as 0.3 μM of Hg2+. The porphyrin compounds 10a-e show promise in sensing a several metal ions of interest including, mercury, cadmium, zinc and silver.
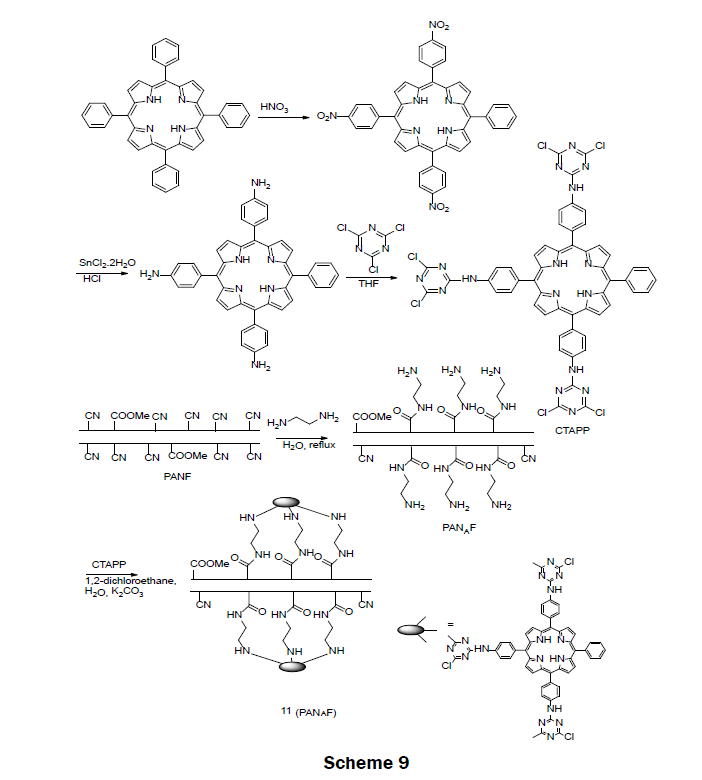
A Porphyrin-Functionalized Polyacrylonitirile FiberIn 2015, a porphyrin-functionalized polyacrylonitirile fiber (CTAPP-PANAF) Hg2+ ion sensor was prepared (Scheme 9) [24]. The sensor reagent was prepared in two steps. First, commercially available 5,10,15,20-tetraphenylporphin (TPP) was functionalized by reacting with concentrated nitric acid followed by reduction with SnCl2·2H2O/HCl to synthesize 5,1015-tris[4-aminophenyl]-20- phenylporphyrin (TAPP). The tris-amino porphyrin TAPP was then reacted with 1,3,5-trichlorotriazine in THF to afford 5,10,15-tris[4- (3,5-dichlorotriazinyl)aminophenyl]-20-phenylporphyrin (CTAPP). The solid sensor 11 was obtained by immobilizing CTAPP onto the aminated polyacrylontirile fiber (PANF). The aminated fiber was prepared by a reaction of ethylenediamine and dried PNAF under reflux conditions. When immersed in a 1 × 10-3 M Hg2+ solution, the fiber changed color from red-brown to dark-green. A LOD was reported as 1 × 10-6 M. The fiber proved to be selective for mercury over Pb2+, Ba2+, Cd2+, Ag+, Zn2+, Cu2+, Ni2+, Co2+, Fe3+, Mn2+, Cr3+, Ca2+, Al3+, and Mg2+ even when the other metals were present at an order of magnitude larger concentration than the mercury ion. The fiber was very reusable; after 50 iterations of absorption-desorption the fiber still responded to mercury ions; however, the response time for the fiber was slowed. For very low concentrations of mercury (1 × 10-7 M) the fiber needed to be incubated in the mercury solution for 24 h. Less incubation time was necessary for larger concentrations. The usability of the fiber was examined with water from the Weijin River; a mercury spiked real water sample was visually detected with a concentration of 1 × 10-5 M Hg2+.
This review describes the progress in the development of porphyrin solid state sensors specific for the detection of Hg2+ ions during the past 10 years (2006-2015). Table 1 summarizes the porphyrin used to sense mercury ions, the immobilization method used to attach the porphyrin onto a solid support, the spectroscopic method used for detection, the linear range, and the LOD. We first address the construction of the solid state sensor by detailing synthetic route to the porphyrin sensing reagent, and how the sensing reagent is associated with the solid support. We then characterize the sensor by reporting the LOD, dynamic range and interferences of which were observed for the sensor. Finally, as appropriate, we identify extensions of the work. Despite some promising advancements in the past ten years, these solid sensors face challenges including limited stability in working conditions, and leaching of the porphyrin unit from the solid-state sensor. Looking ahead, designing and synthesizing new solid mercury ion sensor has a huge development space for researchers to address the issues mentioned above.
DRF and MZ acknowledge support from The Welch Foundation Departmental Grant (AN-0008), and Michael A. Janusa, Chair and Professor of Chemistry and Biochemistry at SFASU. MZ acknowledges support from RCA grant at the Stephen F. Austin State University Research Enhancement Program.