ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ana Francisca Rozin Kleiner1, Manuela Galli2, Paula Teixeira Fernandes3, Chiara Rigoldi4, Aline Araújo do Carmo5, Ricardo M. L. Barros6
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This study aimed to investigate the possible spontaneous recovery in postural control in post-stroke survivors during quite standing at different periods from stroke event, using the center of pressure sway analysis (the antero-posterior and median-lateral direction pattern analysis). Twelve participants in the chronic post-stroke phase and twelve age-matched healthy subjects were barefoot and were instructed to maintain an upright standing position for 60s on a force platform. The data were collected in two conditions: eyes open and eyes closed. The stroke participants were evaluated in 3 different periods. The time and frequency domain analyses of center of pressure signal were performed in order to obtain center of pressure descriptors in both the antero-posterior and mediolateral directions. Statistical analysis was performed to compare groups, periods and conditions. The results pointed out a worst postural control when the visual feedback is off for pathological group: patients in the post-acute phase of stroke tend to rely more on visual information for postural control. The spontaneous balance recovery in individuals with post-acute stroke is characterized by increased visual dependency compared to normal subjects.
Keywords |
| stroke; posture; visual input; proprioception; longitudinal study; neuroscience. |
INTRODUCTION |
| The ability to remain standing during active movements or adverse stimulation requires the integrity of the sensorimotor system of postural control [1]. Sensory impairments are common after stroke, occurring in approximately 60% of people suffering from stroke [2, 3], including tactile and proprioception [3]. Furthermore, sensory dysfunction in the lower limb after stroke has been related to constraints in standing balance [4]. |
| Impaired postural control is a key characteristic of the mobility problems in stroke patients and is caused by a complex interplay of motor, sensory, and cognitive impairments. Previous studies in stroke patients have identified reduced loading on the paretic lower limb and increased postural sway during quiet standing as well as delayed and disrupted equilibrium reactions [5, 6, 7,8]. Together with a general slowness of information processing, this combination of postural deficits causes slow and inflexible motor behavior during various activities of daily life [9]. The lack of postural stability can lead to falls during hospitalization, both in the acute care and rehabilitation stages, and after discharge [10]. |
| Because of the importance of postural instability in this population, a systematic evaluation of balance is needed to identify specific problems and to organize the optimal rehabilitation program that fits to individual needs. During quiet stance on a flat surface, anterior-posterior (AP) and medio-lateral (ML) center of pressure (COP) sway describe the postural adjustments as the final product of the integration of the ongoing input coming from the sensory systems (visual, vestibular and proprioceptive systems). In a laboratory setting, posturography in quiet standing quantifies the amount of postural sway and weight distribution, and, the computation of specific punctual indexes referred to COP signal should reveal the behavior of postural systems in a pathological contest |
| The measures most commonly used with stroke patients are different parameters extracted from the COP signal in the horizontal plane analyzing it in time and frequency domain. After a stroke, patients have demonstrated a significant increase in sway, weight-bearing asymmetry [11, 12] and exaggerated corrective ankle mechanism as assessed by the analyzing the higher frequency of COP components [13]. However, a powerful characteristic of the postural control system is its ability to deal with peripheral lesions by the use of compensatory strategies. One of the most prominent sources for compensation is the visual information: shifting to a more visually dominated control strategy can compensate for a decrease in sensory information from the lower limbs [12, 14, 15,16, 17]. Changes in motor ability might occur via several mechanisms: restitution, substitution or compensation [18]. Restitution referred to the restoration of function in neural tissue that was initially lost and, consequently, the restoration of the ability to perform movement in the same way before injury. Substitution and compensation include the acquisition by neural tissue of a function that it did not have before the injury and, consequently, the performance of a movement in a new way. |
| Recently, interest in the mechanisms underlying balance recovery following stroke has grown, because insight into these mechanisms is necessary to develop effective rehabilitation strategies for different types of stroke [5, 13]. Studies dealing with the recovery of standing balance from stroke are, however, limited to rehabilitation inpatients with a unilateral supratentorial brain infarction or hemorrhage[13]. In most of these patients, stance stability improves in both planes as well as the ability to compensate for external and internal body perturbations and to control posture voluntarily [5, 13]. Although there is evidence of true physiological recovery of paretic leg muscle functions in postural control, particularly during the first three months post-stroke, substantial balance recovery also occurs in patients when there are no clear signs of improved support functions or equilibrium reactions exerted through the paretic leg. This type of recovery probably takes much longer than 3 months [19, 20]. |
| In this study, we explored the recovery of postural control using the COP sway analysis in participants in the chronic phase of stroke, up to 6 months after the ischemic event, with mild motor deficits. Our aim was to monitor the spontaneous recovery of postural control performed by subjects with stroke during quite standing in different conditions (eyes open and eyes closed) making use of the COP sway analysis in the AP and ML directions, in order to investigate the spontaneous compensations induced in postural control and to analyze if the postural performance occurs in a new way. We hypothesized that subjects with stroke would present excessive reliance on visual input as the most natural compensatory strategy for coping with poor balance at all the stages we acquired: reliance on visual input is a spontaneous learned response and the unique possibility to substitute the maintenance of posture when afferent input from non-visual source are reduced. |
Methods |
Subjects |
| The present study was approved by the Ethics Research Committee of the University Ethics (protocol No. 319/2011) and the volunteers gave written informed consent to participate. Before the experiment, all participants gave their informed consent to participate to the study and all investigations were performed in conformity with the ethical and humane principles of research. |
| Twelve participants in post-stroke were analyzed. The average characteristics of the participants were age: 62.83 ± 6.86 years; body mass: 69.50 ± 13.96 kg and height: 1.68 ± 0.06 m; seven males and five females; post -injury time: 6.1 ± 2.8 months after stroke. Out of an inception cohort of 12 stroke patients (viz. data from two patients were excluded due to missing values). The inclusion criteria were: aged between 40 and 75 years; post-stroke time less than 12 months; affected by only one stroke (ischemic); presence of gait alterations. The subjects were evaluated three times with an interval of three months: the first trial at the 6.1 ± 2.8 months after the stroke event (S1); second trial at the 9.17 ± 0.68 months after the stroke (S2); and, third trial at the 12.40 ± 0.6 months after the stroke (S3). In each trial the postural control in the eyes open (EO) and eyes closed (EC) conditions were evaluated. |
| In order to compare these data with a normative reference, we considered an age matched group of healthy subjects (CG) from our database, previously collected for other studies. The CG was composed by 12 healthy adults (5 females and 7 males) and the average characteristics were: age = 63.58 ± 6.95 years; body mass = 73.08 ± 14.31 kg; height = 1.69 ± 0.05 m. The selection criteria for this group were no signs of any orthopedic or neurological diseases or disorders, no impairment of somatosensory, hearing, vestibular and uncorrectable visual functions and no developmental disabilities. |
Procedure |
| The participants were instructed to maintain an upright standing position for 60s on a force platform (Kistler 9286BA) with arms at their sides and feet positioned over sketches representing the foot with an angle of 30º respect to the AP direction. The data were collected into two conditions. In the first one, the participants were asked to maintain an upright standing position with eyes open (OE), looking at a figure positioned 1.5m faraway, in the patient's direct line of sight. In the second condition, the participants were requested to keep their eyes closed (EC). One trial per condition was collected. |
| Participants were requested to sit for a period of about 120s after the completion of each condition in order to rest and to cancel fatigue effects, during the 3 evaluation periods: S1, S2 and S3. |
Data Processing |
| An algorithm developed in Matlab (Mathworks Inc., Natick, USA) was used to filter the raw data and to calculate the COP descriptors. This algorithm was developed to perform three functions: the first was to filter the raw data; the second the data normalization and the last the COP variables calculation. These procedures are detailed bellow. |
| Data Filtering: during the data collection the force platform signals were sampled at 100 Hz and the cutoff frequency of the low-pass filter was chosen after a residual analysis [21]: low-pass filtered at 6 Hz using a dual-pass second-order Butterworth digital filter, After this procedure, the first 10s were considered as an adaptation period and the last 10s were considered as a fatigue period of the 60-s time series and were discarded for the data analysis after the filtering processes. Tasks that involve disturbances to the posture do not require a long duration; a few seconds before and after the perturbation are enough to verify the alterations and the stability of the COP [22]. For stroke patients there is a huge variation of the oscillation during the first second of trial and the last second. According to the patients evaluated during this study, they stood very tired and afraid to fall during the last 10 seconds of the acquisition, especially during the eyes closed condition. That's why we decided to cut off the 10 last seconds of these trials. |
| Data Normalization: in order to avoid the influence of the participant's height on these results, the variables were normalized to participants height [23]. Moreover, the mean position of the COP is not of interest, as it is simply dependent on the absolute position of the subject on the force plate, which, in general, is not controlled. Thus, it is a common procedure to remove the mean position of the COP signal before any analysis procedure. A simple way to remove the tendency of the COP signal is to use the function detrend from the Matlab. |
| COP variables calculation: the COP displacements were computed in the antero-posterior (AP) and medio-lateral (ML) directions and then the time and frequency domain analysis were performed. A complete description of the algorithms appears in a previous study (for more details see 24). The following variables were calculated: Total displacement of sway (DOT): length of COP trajectory on the base of support; this index is related to the energy consumption. |
| COP area: this variable area estimates the dispersion of the COP data through the calculus of the statokinesigram area. There are different ways to calculate this area, and one of the most common is through the statistical method of analysis of the principal components. Using it, it is possible to calculate an ellipse that contains a certain percentage (for example, 95%) of the COP data, being the two axes of the ellipse calculated through the measures of the COP signals dispersion. This index is related to the energy consumption. |
| Range of COP displacement in AP (COPap) and ML (COPml) directions: the difference between the maximum and minimum COP displacement for each direction |
| Root mean square for AP (RMSap) and ML (RMSml) directions: dispersion of COP displacement from the mean position during a time interval. Higher is the RMS highest is the internal perturbation and the need of postural adjustments. |
| Total mean velocity (TMS): The TMV is calculated through the displacement of the total sway of the COP in both directions divided by the total duration of the trial. Higher is the velocity highest is the postural perturbation and the falls risk. |
| Mean velocity for AP (VMap) and ML (VMml) directions: to determine how fast were the COP displacements in the AP and ML directions. These variables were calculated to quantify the direction of the perturbation. COP power frequency in 80% (F80) of the spectral power frequencies for the AP and ML directions: the Fourier analysis allows the decomposition of any signal as a sum of the sine and cosine functions with different amplitudes, frequencies, and phases. Thus, it is possible to obtain information about the frequencies that compose a signal. The predominant frequency or peak frequency is that with the highest amplitude among all frequencies that compose the spectrum. Baratto et al.[25] suggested that the frequency band with 80% of the spectral power is the one that best characterizes the modifications on the postural control system. The frequency analysis should explain the dynamics with which the COP signal is generated in order to maintain the equilibrium. |
Statistics |
| The data did not present normal distribution (Kolmogorov-Smirnov test), so the non-parametric statistics were applied. First the comparisons between EO and EC for each group were performed by the Wilcoxon signed-rank test. The comparisons between Control Group and Stroke for each period of testing were performed by the Mann-Whitney Test. This test performed comparisons between control group and each stroke evaluation (S1, S2 and S3) for each condition (EO and EC) separately. Finally, the Wilcoxon signed-rank test performed comparisons between each stroke evaluation (S1, S2 and S3) for each condition (EO and EC) separately. All the statistical tests were analyzed in The SPSS ® software (SPSS for Windows, version 19.0) performed the statistical analysis with a significant level of α<0.05 for all tests. |
RESULTS |
| The Figure 1 is an example of one stroke performance statokinesigram along the six month interval (from S1 to S3) under EO and EC conditions in comparison with aged matched healthy control subject. In this analysis (the map of COP AP versus COP ML), the healthy subject and the participant with stroke produced different patterns of COP sway under EO and EC conditions. In both conditions, EO and EC, for the first trial (S1 – Figures 1c and 1d), the participants with stroke presented higher variability in both COP directions in comparison with the healthy control (Figures 1a and 1b). From S1 to S3, a COP variability reduction in stroke subjects can be seen under eyes open conditions, however the same effect is not clear when observing the EC condition. However, in the EO conditions during the second (S2 – Figure 1e) and third (S3 – Figure 1g) trials, the participant with stroke presented a very similar pattern as the healthy control subject. However, in the EC condition in the trials 2 (S2 – Figure 1f) and 3 (S3 – Figure 1h) the stroke patients still presented higher variability in both COP directions in comparison with the healthy control, the individuals with stroke displaced the mean COP location several times as in the first trial. |
| Statistical differences under EO condition were observed between groups in trial 1 in all variables: DOT (Figure 2a), Area (Figure 2b), COPap (Figure 2c), COPml (Figure 2d), RMSap (Figure 2e), RMSml (Figure 2f), VMap (Figure 3a), VMml (Figure 3b) and TMV (Figure 3c); and differences trial 1 and trial 2 where seen in DOT, VMml and TMV within the stroke group. Moreover, differences in EC condition were also observed between control group and stroke group in trial 1 in all variables: DOT (Figure 2a), Area (Figure 2b), COPap (Figure 2c), COPml (Figure 2d), RMSap (Figure 2e), RMSml (Figure 2f), VMap (Figure 3a), VMml (Figure 3b) and TMV (Figure 3c); differences between trial 1 and trial 2 were also seen in all variables: DOT (Figure 2a), Area (Figure 2b), COPap (Figure 2c), COPml (Figure 2d), RMSap (Figure 2e), RMSml (Figure 2f), VMap (Figure 3a), VMml (Figure 3b) and TMV (Figure 3c) within the stroke group; and differences between trial 1 and trial 3 were found in DOT (Figure 2a), Area (Figure 2b), COPml (Figure 2d), RMSap (Figure 2e), RMSml (Figure 2f), VMap (Figure 3a), VMml (Figure 3b) and TMV (Figure 3c) within the stroke group. There were significant differences between the EO and EC conditions for trial 1, trial 2 and trial 3 for COPap (Figure 2c) and VMap (Figure 3a); VMml (Figure 3b) for stroke group trial 1 and trial 3; and, just for stroke group trial 1 for RMSap (Figure 2e). |
| The Figure 4 illustrates the COP spectral power frequencies variables for each trial and condition (EO and EC). Significant differences between groups for the EO condition were found for: between CG and S3 for F80ML (Figure 4b); and between S1 and S3 for F80ML (Figure 4b). Moreover, significant differences between groups for the EC condition were found for: between CG and S1 for F80AP (Figure 4a); between CG and S2 for F80AP (Figure 4a); and between CG and S2 for F80AP (Figure 4a). There were significant differences between the EO and EC conditions for all the trials in the F80ap within pathological group. No differences between conditions were found for control group. |
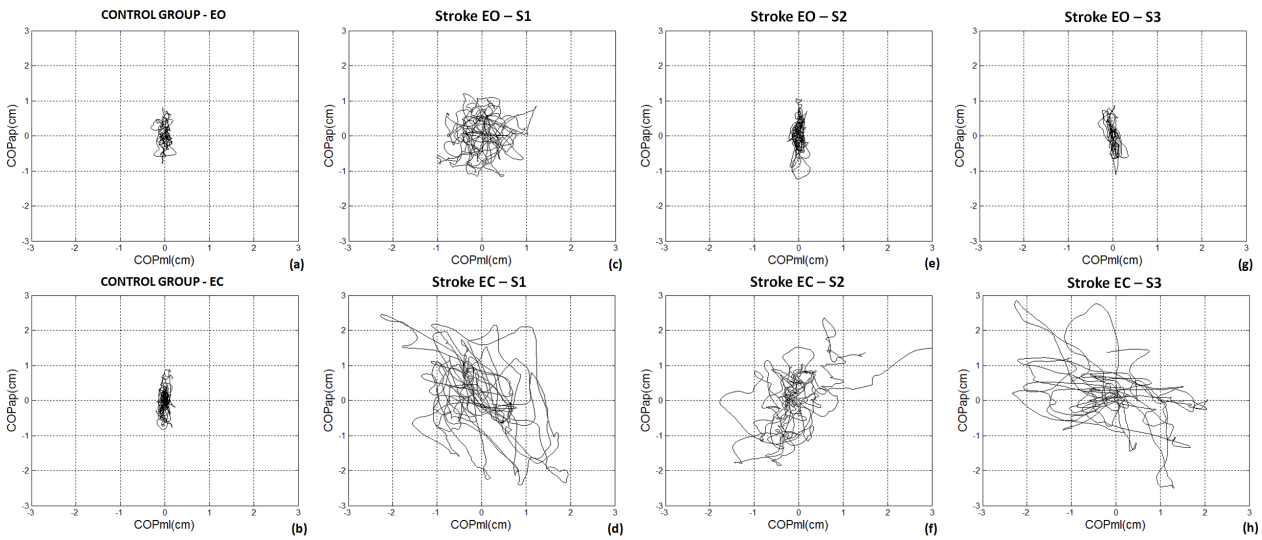 |
| Fig 1. Representative statokinesigram from one healthy control subject and one stroke individual along 6 months follow up under EO and EC conditions. Legend: S1 = first trial; S2 = second trial; S3 = third trial; EO = eyes open; EC = eyes closed. |
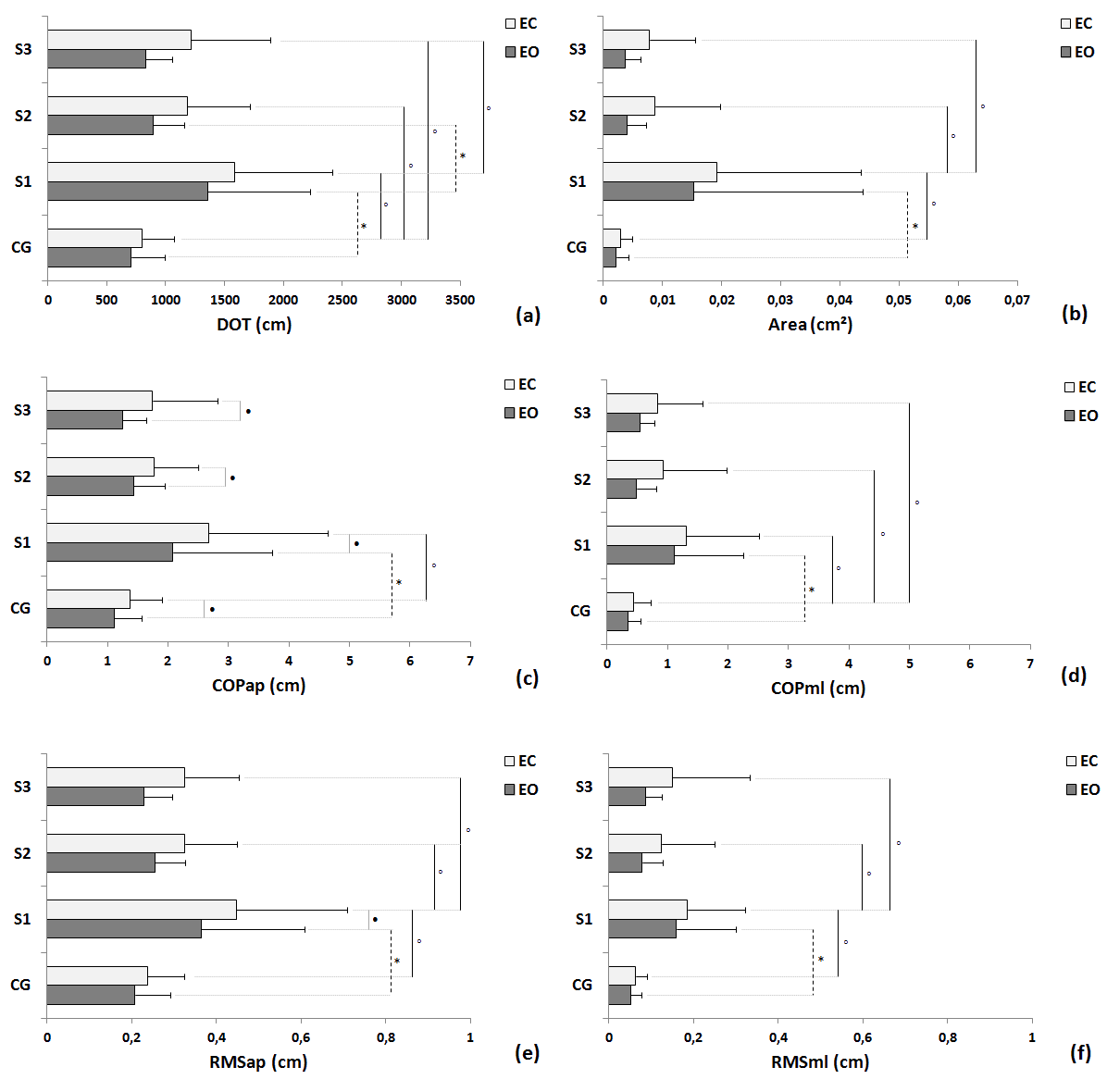 |
| Fig 2. Comparisons of control group (CG) and participants with stroke (S) in the three trials in terms of mean, standard deviation and statistical values for the EO and EC conditions of follow variables: (a) total displacement of sway (DOT); (b) area; COP displacement for AP direction (COPap – Figure 2c) and for ML directions (COPml – Figure 2d); and root mean square for AP (RMSap – Figure 2e) and ML directions (RMSml – Figure 2f). Legend: * = p< 0.05 between EO condition; âÃâÃâ¹ = p< 0.05 between EC condition; âÃâà= p<0.05 between EO and EC condition; S1 = first trial; S2 = second trial; S3 = third trial. |
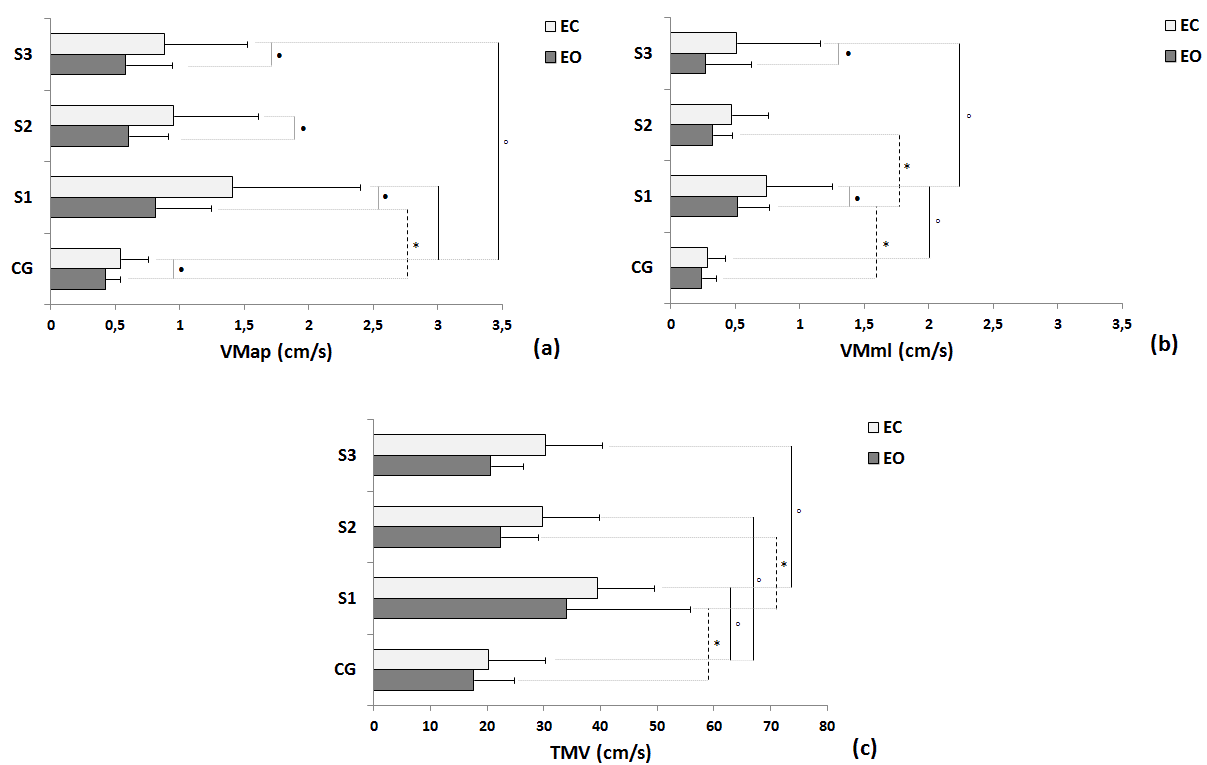 |
| Fig 3. Comparisons of control group (CG) and participants with stroke (S) in the three trials in terms of mean, standard deviation and statistical values for the EO and EC conditions of follow variables: (a) Mean velocity for AP (VMap - a) and ML (VMml - b) directions, and Total mean velocity (TMV – c). Legend: * = p< 0.05 between EO condition; âÃâÃâ¹ = p< 0.05 between EC condition; âÃâà= p<0.05 between EO and EC condition; S1 = first trial; S2 = second trial; S3 = third trial. |
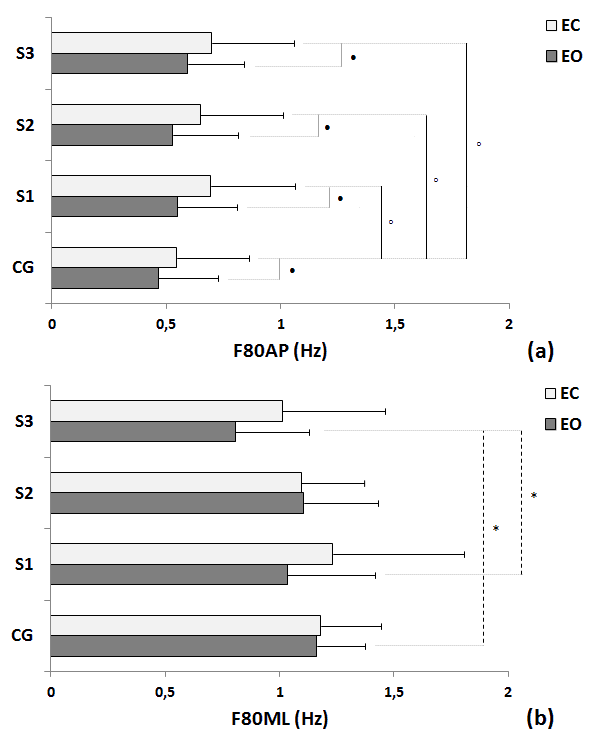 |
| Fig 4. Comparisons of control group (CG) and participants with stroke (S) in the three trials in terms of mean, standard deviation and statistical values for the EO and EC of COP power frequency in 80% for the AP (F80AP - a) and ML (F80ML – b) directions. Legend: * = p< 0.05 between EO condition; âÃâÃâ¹ = p< 0.05 between EC condition; âÃâà= p<0.05 between EO and EC condition; S1 = first trial; S2 = second trial;S3 = third trial. |
DISCUSSION |
| This work studied the spontaneous postural control recovery performed by persons with Stroke under different conditions (eyes open and eyes closed) in a follow-up period without rehabilitation treatments. The results permitted us to analyze and evaluate the compensatory functions used in maintaining the vertical posture in the case of a pathology that makes unreliable the use of vestibular and proprioceptive afferent input. Our experimental design allowed us to evaluate the spontaneous plasticity in reweighting the contributions of proprioceptive, vestibular and visual input as well as their integration in maintaining quiet standing, making the use of the COP sway analysis in time and frequency domain, considering both the antero-posterior (AP) and median-lateral (ML) directions. |
| Based on the time domain analysis we observed statistical differences, considered as improvements since the variables approach to normal pattern, concerning the sway length and COP displacements in both directions under EO and EC conditions in the 3 periods of acquisitions, documenting happens in spontaneous postural control recovery. Along the recovery period, stroke participants showed higher values of the computed time domain parameters than control group in both conditions and directions: postural control for pathological group resulted compromised and with a higher energy cost, as confirmed for example by the higher values of DOT and Area. |
| Comparing EO and EC conditions in pathological group at all the analyzed periods, time domain analysis pointed out a worst postural control without the visual feedback (as reported by COPap, VMap and VMml); otherwise controls did not show statistical differences between conditions, showing adaptation in sensory information organization when one of the systems was suppressed: patients in the post-acute phase of stroke tend to rely more on visual information for postural control in both planes, as reported in several works [12, 13, 17], evidencing the loss of the ability to switch the mode. |
| “simpler” explanation for the increased visual dependence in patients with stroke is a disease non-specific strategy to compensate for the loss or distortion of other sensory input [5, 12, 13, 17]. As literature reported, spontaneous recovery happens as the reweighting of the incoming input from the three systems governing the postural control, but the CNS seems to drive the easiest way, excluding the possibility to restore autonomously the residual proprioceptive capacities: removing visual information may be initially disruptive to posture until remaining sensory inputs can be reorganized [26]. Our results are in line with literature: deprivation of vision in EC condition provokes increased COP amplitudes and velocities during the unperturbed standing. In control group the absence of visual input in EC acquisitions did not provoke a perturbed COP signal as in stroke survivors: the intact proprioceptive system, together with vestibular system, acted in maintaining vertical posture also without visual information, in this sense the CNS acted reweighting the gain of the three postural systems |
| Otherwise, the participants with stroke in the same condition could not count on the input coming from the distorted proprioceptive system, that induced the individuals with stroke to unconsciously increase the gain of the vestibular contribution in maintaining vertical posture, as reported by the increasing of the COP amplitudes and velocities and, consequently, the risk of falls increased for the pathological group. Working on the restoring of proprioceptive residual capacities could lead the patients to another degree of autonomy and probably to a better degree of functional recovery, decreasing also the gain of vestibular contribution in quiet standing and consequently decreasing the risk of falls. Concerning the results we obtained in frequency domain in AP direction, we reported no differences in F80ap index between the three periods considered both in EO and EC conditions for the stroke survivors group. The frequency analysis showed higher frequency for pathological group in comparison with control group in both conditions confirming not synergic muscle actions in postural control without the visual input. Ankle strategy is the main mechanism in maintaining vertical posture and it is regulated by the coordination between triceps surae and tibialis anterior activations: post-stroke survivors lack this synchronized activations due to the impairments derived from a decrease in muscle spindles activation threshold, spasticity or other disorders of sensory information organization that could underlie a distorted representation of the body in space [17]. The differences between OE and CE within the pathological group pointed out their unreliable proprioceptive input in maintaining vertical posture: the higher frequency showed in CE condition evidenced the continuous adjustments induced by the disrupted ankle strategy, resulting in an exaggerated corrective ankle mechanism, due to the various and mixed components affected balance in post-stroke participants. |
| These results indicated us that the spontaneous recovery of the residual proprioceptive capacities did not take place during the three acquired periods. Such patients exhibit excessive reliance on visual input and they lose the ability to use somatosensory and vestibular input correctly [17] but probably removing visual information and giving to the remaining sensory inputs the possibility to be reorganized should be an attempt to perform, even if at the beginning it should be disruptive. The rehabilitation could “hardly” act, taking into account that spontaneous recovery works on visual input and guiding the individual to take advantage of residual proprioceptive abilities, even if it should be initially more disruptive. We have implemented an automatic text detection technique from an image for Inpainting. Our algorithm successfully detects the text region from the image which consists of mixed text-picture-graphic regions. We have applied our algorithm on many images and found that it successfully detect the text region. |
CONCLUSION |
| Balance recovery in individuals with stroke without intervention is characterized by a reduction in postural sway and instability during the EO condition. But there is no reduction in visual dependency. Patients in the post-acute phase of stroke tend to rely more on visual information for postural control in both planes in all the analyzed sessions. Deprivation of vision provokes increased COP amplitudes, velocities and in the time and frequency domain analyses, especially for the AP direction, during unperturbed standing. Moreover, the most important impact on postural control recover should be reached in the first 6 months. Our future studies will focus in the intervention of this patients during this period focusing the standing with eyes closed should be interesting, since others sensorial systems (e.g. vestibular and kinesthetic) can be training and, also the individual with stroke can better explore new control mechanisms to the restoration of support functions and equilibrium reactions of the paretic leg. |
ACKNOWLEDGEMENTS |
| The authors of this paper are very grateful to the participants, especially the ones with stroke, who generously gave their time to assist with this research. We would also like to thank the Conselho Nacional de DesenvolvimentoCientífico e Tecnológico – CNPq and CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (process: BEX 11241/13-6) for the financial support provided to conduct this research. |
References |
|