e-ISSN: 2321-6182 p-ISSN: 2347-2332
e-ISSN: 2321-6182 p-ISSN: 2347-2332
Yogendr Bahuguna*, Suhaib Zaidi, Neeraj Kumar, and Kailash Rawat
Division of Pharmaceutical Sciences, SGRRITS, Patel Nagar, Dehradun- 248001, Uttarakhand, India
Received: 23 September 2014 Accepted: 11 February 2015 Published: 21 March 2015
Visit for more related articles at Research & Reviews: Journal of Pharmacognosy and Phytochemistry
In the few decades, there has been exponential growth in the field of herbal medicines. Most of the traditional system of medicine is effective but they lack standardization. So there is a need to develop a standardization technique. Standardization of herbal formulation is essential in order to assess the quality, purity, safety and efficacy of the drug. Dabur Triphala Churna is used for immune system stimulation, improvement of digestion, relief of constipation, gastrointestinal tract cleansing, relief of gas, treatment of diabetes and treatment of eye disease. The present research study deals with standardization of Dabur Triphala Churna [i.e. Emblica officinalis (Garetn.) (Amla), Terminalia bellirica (Gaertn.) Roxb. (Baheda) and Terminalia chebula (Retz.) (Harada)]. The standardization of this formulation, the organoleptic characters, physical properties, the various physico-chemical properties such as moisture content, ash values, extractive values were carried out. Heavy metal content studies were also carried out to ascertain the quality, purity and safety of this herbal formulation.
Standardization, Herbal Formulation, Dabur Triphala Churna, Physico-chemical parameters.
Nature always stands as a golden mark to exemplify the outstanding phenomena of symbiosis. Today about 80% of people in developing countries still relay on traditional medicine based largely on the different species of plants for their primary health care. About 500% of plants with medicinal uses are mentioned in ancient literature and 800 plants have been used in indigenous system of medicine. The various indigenous system such as Ayurveda, Siddha, Unani use several plant species to treat different ailments [1,2,3]. Herbal medicines make up an important component of the trend toward alternative medicine. A Harvard study recently found that one in three respondents acknowledged use of at least one alternative therapy within the past year. Extrapolated, these findings suggest that up to $13.7 billion were spent in 1990 alone for these treatments [4]. Tyler defines herbal medicines as "crude drugs of vegetable origin utilized for the treatment of disease states, often of a chronic nature, or to attain or maintain a condition of improved health"[5]. Current demands for herbal medicines have resulted in an annual market of $1.5 billion and increasingly widespread availability [6].
Historically, herbal medicines have played a significant role in the management of both minor and major medical illnesses. One example is foxglove, which contains cardiac glycosides, and serves as a classic treatment for congestive heart failure. Even now, physicians still use many drugs that possess botanical origins. Huxtable notes that one-quarter of the prescriptions currently written in the United States are for plant products, while one quarter is for agents based on botanical compounds. The therapeutic potential of herbal medicines cannot be ignored and is highlighted in the three examples provided next [7].
• They have large amount of use.
• They have better patient tolerance as well as acceptance.
• The medicinal plants have renewable source of cheaper medicines.
• Improvements in the quality, efficacy and safety of herbal medicines with the development of science and technology.
• Prolong and apparently uneventful use of herbal medicines may offer testimony of their safety and efficacy.
• They are cheap in cost.
• They are not harmful.
• They are more effective than any synthetic drug.
• Throughout the world herbal medicines have provided many of the most potent medicines to the vast arsenal of drugs available to modern medical science, both in crude form as well as a pure chemical upon which modern medicines are constructed[8,9].
The quality control of herbal crude drug & formulation is important in justifying their acceptability in modern system of medicines. Standardization of synthetic drugs offers no problem with very well defined parameters of analysis. It is not uncommon to have as many as five or more different herbal ingredients in one single formulation. The batch to batch variation starts from the collection of the raw materials itself in absence of any reference standard for identification. WHO has emphasized the need to ensure quality control of medicinal plants products by using modern techniques and by applying suitable standards and parameters. Standardized products and services are valuable. User confidence builder’s being perceived as:
• Safe
• Healthy
• Secure
• High quality
• Flexible
Standardization brings important benefits to business including a solid foundation upon which to develop new technologies and an opportunity to share and enhance existing practices. Standardization also plays a pivotal role in assisting Governments, Administrations, Regulators and the legal profession as legislation, regulation and policy initiatives are all supported by standardization [10, 11, 13].
Sample Name-
Main Constituents
• Senna leaves
• Haritaki
• Liquorice
• Constipation
• Acidity
• Headache
• Colour
• Odour
• Taste
• Moister content
• Total ash
• Acid insoluble ash
• Water soluble ash
• Water soluble extractive
• Alcohol soluble extractive
• Powder fineness
• Bulk density
• Tap density
• Angle of repose
• Compressibility
• Hausner ratio
Establishing the safety pertaining to Heavy metals.
The polyherbal formulation is studied for organoleptic characters like color, odour and taste using the sensory organs of our body.
10 g of the sample (without preliminary drying) was weighed and placed in a tarred evaporating dish. It was dried at 105°C for 5 hours and at 1 hour interval until difference two successive weighing corresponded to not more than 0.25%.
About 2 to 3 g of sample was accurately weighed in a tarred silica dish at a temperature not exceeding 450°C until it was free from carbon. Then it was cooled and weighed. The percentage of total ash was calculated with reference to the air dried drug.
The total ash obtained was boiled for 5 minutes with 25 ml of dilute hydrochloric acid; the insoluble matter obtained was collected on an ash less filter paper, washed with hot water and ignited to constant weight. The percentage of acid insoluble ash was calculated with reference to the air dried drug.
The ash obtained in the determination of total ash was boiled for 5 minutes with 25 ml of water. The insoluble matter was collected on an ash less filter paper and washed with hot water. The insoluble ash was transferred into a tarred silica crucible and ignited for 15 minutes at a temperature not exceeding 450°C. The weight of the insoluble matter was subtracted from the weight of the total ash. The difference in weight was considered as the water- soluble ash was calculated with reference to the air dried drug.
5 g of test sample was weighed and macerated with 100 ml of chloroform water in a closed flask for twenty-four hours, shaking frequently during six hours and allowing standing for eighteen hours. It was filtered rapidly, taking precautions against the loss of solvent. 25 ml of the filtrate was taken and evaporated to dryness in a tarred flat bottomed shallow dish at 105°C, to constant weight and weighed the percentage of water soluble extractive was calculated with reference to the air dried sample.
Procedure for water soluble extractive was followed for the determination of alcohol soluble extractive but 90% ethanol was used instead of chloroform water.
It is the ratio of given mass of powder and its bulk volume. It is determined by transferring an accurately weighed amount of powder sample to the graduated cylinder with the aid of a funnel. The initial volume was noted. The ratio of weight of the volume it occupied was calculated.
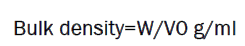
Where, W = mass of the powder, V0 = untapped volume
It is measured by transferring a known quantity (25g) of powder into a graduated cylinder and tapping it for a specific number of times. The initial volume was noted. The graduated cylinder was tapped continuously for a period of 10-15 min. The density can be determined as the ratio of mass of the powder to the tapped volume.
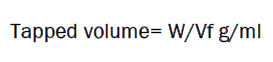
Where, W = mass of the powder, Vf = tapped volume.
It is the propensity of the powder to be compressed. Based on the apparent bulk density and tapped density the percentage compressibility of the powder can be determined using the following formula.
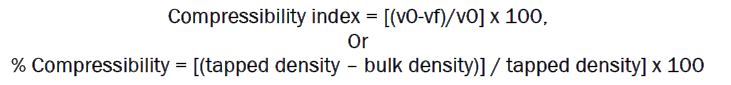
It indicates the flow properties of the powder. The ratio of tapped density to the bulk density of the powder is called Hausner ratio.

The internal angle between the surface of the pile of powder and the horizontal surface is known as the angle of repose. The powder is passed through funnel fixed to a burette at s height of 4 cm. A graph paper is placed below the funnel on the table. The height and the radius of the pile were measured. Angle of repose of the powder was calculated using the formula
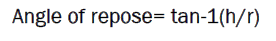
Where, h=height of the pile, r = radius of the pile
The powder sample of Dabur Triphala Churna was weighed to about 5g and immersed in 100 ml of water in a beaker. The beaker was closed with aluminum foil and left behind for 24 hours in room temperature. Later the supernatant solution was decanted into another beaker and the pH of the formulation was determined using a calibrated pH meter.
For Cadmium
For Bismuth

For Lead
Determination of Organoleptic Characters
Physico Chemical Standards
Ash Values
Moisture Content/ Loss on Drying
Extractive Values
Qualitative Analysis
Determination of Physical Characteristics of Powder
Bulk Density & Tap Density
Carr’s Index & Hausner Ratio
Angle of Repose
Determination of pH
Heavy metal test
For Cadmium
For Bismuth
For Lead
The total ash value is an indicative of total amount of inorganic material after complete incineration and the acid insoluble ash value is an indicative of silicate impurities, which might have arisen due to improper washing of drug. The loss on drying value obtained is an indicative of amount of moisture content present in the drug. The extractive values names water soluble and alcohol soluble indicates the amount of active constituent in given amount of plant material when extracted with respective solvent, values obtained supports the fact that drug is unexhausted which is contrary to lower extractive value.
The results of phytochemical tests indicate the presence glycosides, alkaloids, tannins, saponins and sugars. From the heavy metal test it is concluded that Dabur Triphala Churna is free from heavy metals.
From the all above values, it can be concluded that the quality of Dabur Triphala Churna is “GOOD”.
From the present investigation various standardization parameters such as physicochemical standards like total ash, acid insoluble ash, water & alcohol soluble extractive values, loss on drying, phytochemical analysis, flow properties and safety evaluation were carried out, it can be concluded that the formulation of Dabur Triphala Churna contains all good characters of an ideal Churna and it was found to be harmless, more effective, and economic.
The sample shows satisfactory results, but the efficacy of the products can only be judged by doing the pharmacology of which is suggested as future scope of R & D. The study shows that the contents of formulation presents within the permissible limits as per WHO, all these investigations are not specified in the standard literature such as in pharmacopoeia, which could helpful in authentication of Dabur Triphala Churna. The result of present study will also serve as reference monograph in the preparation of drug formulation.
The authors express their gratitude to Shri Mahant Devendra Dass Ji Maharaj, Chairman, Shri Guru Ram Rai Institute of Technology and Sciences, Dehradun, Uttarakhand, India for providing the facilities necessary to carry out the research work.