ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Nadaf. Y. F1, Renuka. C.G2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The photophysical properties of laser dye molecule (C6) were investigated in various solvents using steady-state and time-resolved fluorescence techniques. Fluorescence peak was observed in various solvents of different polarity function. The emission band observed at 500 nm and absorption band at 457 nm at lower concentrations. Solvatochromic shifts of absorption and fluorescence emission as a function of solvent polarity were analysed and the change in dipole moment of the molecule was observed.The Steady-state and timeresolved fluorescence of molecules in various solvents has been investigated. Time-resolved fluorescence anisotropy measurements show that the rotational dynamics of the probe becomes faster in alcohol. A comparison of the experimentally measured rotational reorientationtimes of coumarin in a given solvents at a particular temperature. The present study has been undertaken to examine the role of polarity, friction experienced by the polar solute in a polar and non-polar solvents. Molecular shape and size are similar but the friction experienced by these probes in alkane and alcohol solvents viscosities were varies by temperature. However, it was observed that molecules rotate faster in alkanethan alcohol.
Keywords |
| steady-state, anisotropy, fluorescence life time |
INTRODUCTION |
| The laser dyes have several applications such as anticoagulants, fluorescence indicator and possess anthelmintic and optical brightness properties [1]. The interest in fluorescent dyes for qualitative and quantitative assays has increased considerably during last 20 years. Sensitivity, simplicity and selectivity of fluorescence based techniques make them particular attractive for vitro and in vitro cellular and molecular biology studies [2]. |
| Dye-sensitized solar cells (DSSCs) have considerable promise for future commercialization due to their highenergy conversion efficiency and low production cost, making them viable alternatives to silicon solar cells. Flat membranes with honey-comb like morphology were prepared on dye coated TiO2 electrode. The quasi-solidstate DSSCs results energy conversion efficiency and commercialization of new generation of solar cells [3]. The improved photovoltaic performance of the DSSCs can be correlated with milling time, the strength of the TiO2 porous films as well as the dye loading of the films. Milling and the subsequent increase of surface area leads to higher dye adsorption on titania films and results maximum power conversion efficiency for plastic-based solar cells [4]. |
| The number of works has been reported on laser dyes in various fields with view, we focus here on the rotational dynamics of coumarin laser dyes in a medium. The nature of rotational motion in solution has been a subject of long-standing interest in physical chemistry because such motions directly reflects the interactions between a solute molecule and its surroundings. For this reason, studies of rotational dynamics provide a useful for exploring the nature of solvent friction and how it influences more complex dynamics, such as chemical reaction [5-8]. |
| The present study reports the rotational dynamic studies of coumarin 6 (C6) using the Steady-state and time-resolved fluorescence technique. The structure expected to affect the rotational reorientation times due to the formation of hydrogen bonds with the solvent. Thus, the structures and structural changes in the solvent environment around the solute in the solvent are not fully understood. Therefore this study investigates the rotational reorientation characteristics. |
EXPERIMENTAL |
| The dye C6 was procured from Aldrich Chemical Co., as shown in Fig. 1. Steady state fluorescence anisotropies were recorded using Hitachi F2000 spectrophotometer and electronic absorption spectra are recorded on Hitachi model U-3200 spectrophotometer. Rotational reorientation times of C6 were measured using steady state depolarization technique [6] in heptane and decanol solvent of spectroscopic grade. |
 |
| The steady state fluorescence anisotropy <r> was measured experimentally and the quantities given by the following equation |
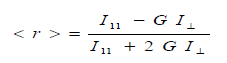 |
| where 11 ïÃÂÃâ° and ïÃÂÞ ïÃÂÃâ° are the polarized fluorescence intensities parallel and perpendicular with respect to the excitation respectively, G is an instrumental factor which corrects for the polarization bias in the detection system is given by HH HV I I G ïÃâ¬Ã½ where IHV is the fluorescence intensity when the excitation polarizer is kept horizontal and IHH is the fluorescence intensity when both the polarizer are kept horizontal.The measurement of <r> involves recording four spectra, two 11 ïÃÂÃâ° and ïÃÂÞ ïÃÂÃâ° and two for G-factor. Each anisotropy measurement was repeated 4-5 times and for every trail, the G-factor was determined. The experiments were performed in the temperature range of 298-342 0K. Rotational reorientation times can be obtained from the measured steady-state anisotropies in the following relation if the decay of fluorescence and decay of anisotropy are single exponential [8-9]. |
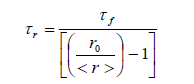 |
| where ro,ïÃÂôf and ïÃÂôr are limiting anisotropy, fluorescence lifetime and reorientation times respectively. The limiting anisotropy ro value was determined by measured steady state anisotropies of the probe molecule in glycerol at low temperature, glycerol having high viscosity under these conditions, when all the rotational motions are frozen. |
| Florescence lifetimes were measured using timecorrelated single-photon counting technique [10] with the gated hydrogen discharge lamp as the excitation source (Edindurgh Instruments, Model EI-199) and the details have been given elsewhere [11]. The excitation wavelength was 420 nm and a cutoff filter GG455 was used to eliminate the excitation light while collecting the emission decay. In both the apparatus, the desired sample temperature was maintained within 0 ïÃâñ1 with the help of temperature controller. |
RESULTS AND DISCUSSION |
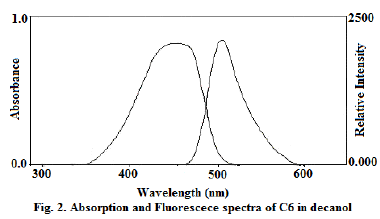
| Absorption and fluorescence emission spectra of molecules were recorded in decanol solvent (Fig. 2). The limiting anisotropy ro was measured by dissolving the solutes in glycerol and measuring steady state anisotropy at low temperature and the values of ro for C6is 0.329. It gives the orientation of the absorption and emission transition dipoles with respect to each other. The axial radii of the molecules were estimated from the Corey- Pauling-Koltum scaled model, long axis was taken along the bond joining benzimidazole group with diethylaminocoumarin and the axis perpendicular to it was taken as the short in-plane axis. van der Waals volumes were calculated using the Edward’s atomic increment method [12]. The estimated axial radius C6 are 9.5 ïÃâô3.7ïÃâô1.9 (axial radii/Å3) and van der Waals volumes are 303 for C6 V/ Å3and the molecule is modeled as asymmetric ellipsoids. |
 |
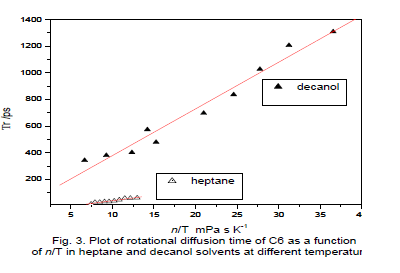
| The reorientation times of C6 decanol as a function of temperature (T), which were obtained from the measured values of ro,ïÃÂôf and ïÃÂôr using equation. Anisotropy <r> values for C6 are in the range of 0.011 to 0.0317 and the ïÃÂôf values are in the range of 2.66 to 2.67 ns in decanol. For heptanes <r> varies from 0.013 to 0.069, ïÃÂôf in the range of 2.29 to 2.37 ns in the temperature ranges from 298 to 342 0K. The rotational reorientation time of a probe in a solvent is in a way an index of molecular friction. Fluorescence decay of both the probes in solvents were single exponential throughout the temperature range used in the study. |
| These probes however, are experiencing more friction in DMSO and n-octanenitrile similar viscosity over temperature [13]. Moog et al. [14] observed that C102 and C153 these probes are similar in size, shape and electronic charge distribution, one would expect nearly identical rotation times and hydrogen-bounding behavior for two solutes. Indeed the rotation times of probes rotates more slowly in alcohol solvents than alkanes.Fig. 3 gives a plot of ïÃÂôr versus of ïÃÂè/T for the both solvents in C6, although linear least-squares fits of ïÃÂôr versus of ïÃÂè/T for both the probe in decaol gave positive intercepts. Positive intercepts indicating that the relationship between ïÃÂôr and ïÃÂè/T is linear in this solvent system. According to general consensus, a polar molecule rotating in a polar solvent should experience dielectric friction. |
 |
CONCLUSIONS |
| Rotational reorientation times of C6 have been measured in alcohol and alkane solvents as a function of temperature. The experimental rotational correlation times are well represented as linear function of ïÃÂè/T. To conclude, the present results on C6 underscore the fact that we are far from having a complete understanding of how it is that the molecular details of solute-solvent interactions translate into friction on solute molecule. One of the goals for undertaking the present study is to find out which aspect is responsible for the observed trend. We may conclude that the dielectric friction experienced by molecules in the solvent medium is responsible for the observed rotation. |
References |
|