e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, DAVPG College, Kanpur 20800, Uttar Pradesh, India
Received: 29/07/2013; Revised: 22/08/2013; Accepted: 23/08/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Studies on charge transfer complexes of group VIII metal acetylacetates with heterocyclic N-bases has been carried out using infrared and proton magnetic resonance analysis. Due to complexation, the effect of stretching frequency of Pt (NO2-acac) 2, have been studied and discussed for the site of interaction and tentative structure for these molecular complexes.
Charge transfer complexes, Infrared, Proton magnetic resonance. Molecular complexes
It has been shown through conductometric [1] and dielectric studies [2] that like aromatics, coordinative saturated, and neutral, monomer Pt (NO2-acac)2 forms molecular complexes with hetrocyclic N-bases in polar and non-polar solvents. Pt (NO2-acac) 2, does not form any adduct with heterocyclic bases. On introducing a NO2 group at ?-position of the acetylacetonate chelate rings, noticeable change in the behavior of Pt (NO2-acac) 2 is observed. [3,4]. The solution of Pt (NO2-acac)2 in carbon tetrachloride, pyridine, quinoline, 2-methyl pyridine, 3-methyl pyridine, 4-methyl pyridine show change in color from yellow to pink. From the conductometric [1] and dielectric studies [2], it has been observed that these bases interact with Pt (NO2-acac)2. However, the site of interaction could not be established from these studies. Hence, in this paper an attempt has been made to isolate the solid adducts of Pt (NO2-acac)2 with pyridine, quinoline, 2-methyl pyridine, 3-methyl pyridine, 4-methyl pyridine.
M (NO2-acac) n was prepared as reposted in the literature [5].
The procedure for the nitration of M (NO2-acac) n with copper nitrate or concentrated nitric acid as nitrating agent was essentially the same as reported in the literature [5]. In place of copper nitrate, Nitrates of Ni (II), Co (II), Al (II) and Fe (II) were also used as nitrating agent in acetic anhydride medium under similar reaction conditions.
Acetylacetone (0.02-0.23 mole) or 1, 3- diphenylpropane – 1, 3 – dione(0.03 mole and acetic anhydride(10-20ml) were placed in a 250 ml Erlenmeyer Flask fitted with a calcium chloride drying tube and stirred magnetically for 15 min at 0°C. Crystals of the metal nitrate (0.01 moles) were pulverized and added in portions to the stirred solution during 30 min. After 1-2 Hours the ice bath was removed and stirring was continued. The contents of the flask slowly dissolved thought an exothermic reaction.
Pressures, which develop inside the flask, were released by occasional lifting of the stopper and undue rise in temperature was checked by dipping the flask in ice – cold water. A colored deposit of nitro derivative was stirred 2-3 hours more at room temperature and then poured into about 200 ml of ice-cold water containing sodium acetate and stirred for a further hour. The precipitated nitro derivative was filtered under suction and washed with water and then with ethanol. After air drying recrystallization from a suitable solvent gave the pure product.
When Nitrates of Metal (0.015 – 0.025 mole) which from more stable chelates were used for the nitration of less stable metal chelates (0.015 mole) in acetic anhydride medium (10-15 ml), the metal atom of the nitrate replaced the ring metal atom of labile chelates during the nitration process. The nitro derivatives of new chelates were obtained as a result of such metal replacement reactions.
Acetylacetone (AR grade) was used after distillation. All the solvents and donors used were AR grade (BDH) and were purified as reported in the literature [5].
Spectral MeasurementsIR spectra were recorded on a Perkins Elmer Model-521 spectrophotometer in 4000-400 cm-1 regions. Samples were prepared in KBr disc. Calibration of the frquency readings was made by polystyrene.
The proton magnetic resonance spectra were taken on a Varian Associates A-60 spectrometer operating at 60MHz. Line positions of the proton resonance of Pt (NO2-acac)2 and carbon tetrachloride or pyridine or quinoline were measured using an internal cyclohexane or TMS resonance reference. Chemical shifts were reproducible to better than 0.3 Hz, the uncertainty being determined by the line width
The pyridine solution of Pt (NO2-acac) 2 was found to acquire a deep red color on keeping for about 20 hours. The residue obtained on evaporation was found to be unstable. The color change occurs only with excess to the base.
Infrared Absorption Spectral Studies on Pt (NO2-acac) 2In the metal acetylacetonate ring (I) where delocalization of II-electron throughout the ring has been invoked in order to account for the pseudo-aromatic character, the M=O bond hence the v (M=O) should be expected to vary with the substituent groups R1, R2 and R3.
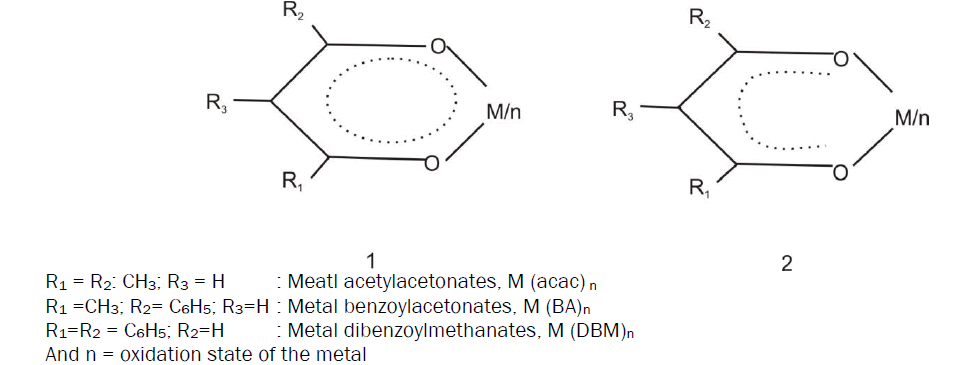
The strength of equivalent pairs of C=C, C=O and M=O bonds have been found to be effective by making the changes at R2 keeping R3=H. Regarding this conclusion some controversial statements were observed. For example, Holtzclaw and Collman [6] have mentioned that the phenyl-substitution in the metal 1, 3-diketonate chelates ring weakened the M=O bond. This was due to the conjugation of M=O bond with the phenyl rings. Simultaneously, Nakamoto, Morimoto and Martel [7] have reported that phenyl substitution slightly strengthens the coordinated bond. Holtzclaw and Collman [6] have mentioned that the phenyl substitution in the metal 1, 3-diketonate chelates ring weakened the M=O bond. West and Riley [8] have observed that the M=O bond strength is related with that of C=O bond. However, the reverse was noted by Bellamy and Branch [9].
The electron withdrawing nitro group at central carbon atom of the Pt(NO2-acac)2 ring experiences almost similar effect as for halogen substituent. The C=C and C=O bonds are weakened; the M=O bond tends to the slightly strengthened whenever it is noticeably effected. However, this description of resonance participation is not an adjustment with the experimental observations. The (C=O) due to the acetyl Group which noticeably decreases, however, suggests limited resonance participation of γ-substituent only with the ligand portion of the chelate ring. Thus, it appears as if the γ-substituent exerts on combined electron withdrawing resonance and inductive effect along the C=C and C=O bonds. Therefore, the M=O bond strength of the chelates is defined by the electron withdrawing inductive effect of the chelates central metal ion which, at least, may involve insignificant electron withdrawal from the ligand.
The main infrared spectral bands of Pt (acac) 2, Pt (NO2-acac) 2 and adducts with possible assignments are compared in Table 1. The IR absorption bands of Pt (NO2-acac) 2 are shown in Fig. 1 and relevant data with possible assignments are recorded in Table 1. It is observed that main stretching in the IR spectra of Pt (NO2-acac) 2 is due to C=C, C=O and M=O vibrations. A strong band is observed at 1595cm-1 due rocking band are observed at 1395cm-1 and 1010cm-1, respectively. A shoulder at 500cm-1 is due M=O stretching (Table 1).
In the IR spectra of Pt (acac) 2 a strong band at 1590cm-1 is observed. This band is due to C=C and C=O vibrations. Strong bands due to vasy (NO2) and vsym (NO2) are observed at 1530 cm-1 and 1342 cm-1, respectively. The CH3 deformation and CH3 rocking bands are observed at 1395 cm-1 and 1005 cm-1, respectively. A strong band observed at 825cm-1 is due to either the C-N stretching vibration or N-O deformation. A shoulder is observed at 500 cm-1 due to M=O stretching (Table 1).
The IR spectra of Pt (NO2-acac) 2 pyridine adduct (Fig. 2), the v (C=O) and v (C=C) are at 1575 cm-1. The band at 1530 cm-1 due to nitro asymmetric stretching vibration of Pt(NO2-acac)2 disappears and two new bands appear at 1480 and 1435 cm-1. The band due to C-N stretching or N-O deformation vibration at 825 cm-1 appears at the same position with less intensity. A shoulder is observed at 475 cm-1 due to stretching of M=O with less intensity (Table 1).
There are two possibilities regarding the site of interaction in these molecular complexes:
The base may directly attach to the metal.
The base may interact with acetylacetonate chelate ring.
The first possibility of interaction may be neglected on the following grounds:
Pt metal in Pt (NO2-acac) 2 has coordination number six and if the pyridine is attached to the metal, it will acquire coordination number seven which is untenable. The other possibility is the cleavage of one acetylacetonate ring and coordination two pyridine as orientate by ring.
The solution of Pt (NO2-acac) 2 in respective heterocyclic bases was found to acquire a deep red color on keeping for about 2-5 days and the solids obtained were found to be unstable and give smell of the respective bases. The analysis support 1:1 stoichiometry of these adducts. The color change occurs only with comparatively large excess of bases. It is important to note that Pt (acac) 2 is unaffected by pyridine and other heterocyclic bases even after long standing. This observation neglects possibility.
The presence of electron withdrawing –NO2 group at 3-carbon atom of Pt (acac) 2 makes it II-electron acceptor and heterocylic base as n-donor. So, there is strong possibility of n-II interaction in these complexes. It is supported by IR and PMR data.
PMR Studies on the Interaction of Pt (NO2-acac) 2 and Heterocyclic N-BasesThe PMR spectra of a solution of Pt (NO2-acac) 2 (0.1M) in pyridine or quinoline have been recorded in the beginning and after a lapse of several hours. The CH3 proton chemical shifts of Pt (NO2-acac) 2 in different solvents are listed in Table 2. In the same table the proton chemical shift of Pt (NO2-acac) 2 in an inert solvent (CCl4) has been reported. Though, some higher field shift in –CH3 proton on complexation of Pt(NO2-acac)2 with pyridine is expected due to the charge-transfer from n-orbital of the base to a vacant II-orbital of the acceptor chelate ring. But, no appreciable change in it was observed (Table 2). Further, an interesting splitting in –CH3 proton chemical shift after keeping this reaction mixture for about 20 hours was observed. This may be due to the interaction of n-orbital of pyridine with vacant II-orbital of chelate ring, which creates the-CH3 proton in different electronic environment and thus disturbs the symmetry of the chelate rings. In the case of quinoline an appreciable change in the –CH3 proton chemical shift to higher field is observed (+14 Hz) which is according to expectation spitting in the –CH3 proton chemical shift has also been noticed like pyridine.
Structure for the interaction of Pt (NO2-acac)2 with pyridine is given below (Fig 3)
It has been shown by various workers [10,11] that aromatic nitro compounds from highly colored adducts with various bases and the behavior of Pt (NO2-acac) 2 is somewhat similar to the aromatic nitro compounds, thereby providing support on pseudo-aromatic character to these chelate rings. Although Dielectric, Conductometric, treatment of hard soft acid and base studies, towards solving the problems of aromaticity in metal acetylacetonates and nature of bonding in molecular complexes of M(NO2 – acac)n and with heterocyclic N-bases reported [12-24] in recent years.
The present study mainly focus on the isolation of solid adduct of heterocyclic N-Bases with Pt(NO2-acac)2. The infrared and proton magnetic resonance spectral studies have been helpful in the characterization of solid adduct. Due to complexation the effect of stretching frequency of Pt (NO2-acac)2 has been studied and discussed. It is also concluded from present study, a probable structure for the interaction of Pt(NO2-acac)2 with heterocyclic bases have been proposed.