ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Rasmika Patel1, Mayank Shah2, Amin Hirani2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Flame retardant polyurethanes were synthesized by reacting a diphosphorus based monomer, bisphenol- A bis (hydroxy phenyl phosphonate) (BABHPP) with different diisocyanates. Polyurethanes were characterized using chemical and instrumental analysis techniques like elemental analysis, FTIR, GPC techniques. Polyblends were prepared by mixing the polyurethanes with conventional DGEBA resin in different proportions and used for casting and coating purposes. Nanocomposites were prepared using organo modified montmorillonite clay. Thermal, flame retardant, impact, tensile properties of nanocomposites, various coating performances and properties were determined. Data reveals that polyblends and nanocomposites show better properties compare to the neat polyurethanes.
Keywords |
| Polyurethanes, Flame Retardant, Epoxy, Blends, Nanocoatings |
INTRODUCTION |
| The unique combination of the properties of toughness, flexibility, solvent resistance, ability to custom tailored has led to the widespread and continually increasing use of polyurethanes for many applications [1-2].Along with the improvement in these properties, for certain commercial applications there is also requirement of flame retardant properties [3].Flame retardant polymers containing phosphorus moiety proved to be non-toxic compared to the halogen based systems [4]. Therefore, to produce a cost effective flame retardant product [5], it was thought to blend the phosphorus based polyurethanes with the epoxy resin. Epoxy resins have shown wide use for their superior adhesion, low shrinkage and chemical resistance properties [6] .But the conventional epoxy is inherently flammable and possess low toughness properties. On the contrary, polyurethanes have better toughness, processibility and setting characteristics compare to the epoxy resin. Thus, the blends of these two types of polymers, epoxy resin and polyurethanes, will give the combined properties of the individual components[5]. Recently nanoclay has been used extensively to improve various properties of coatings[7].Similar way of improvement in mechanical properties of polymer nanocomposites has been reported by many workers [8] at low loading of silicates. |
| In the present work, polyurethanes have been synthesized from a monomer containing diphosphorous moiety and blended with conventional diglycidyl ether of bisphenol-A resin. The polyblends were used for nanocomposite and nano coating applications. Evaluation of mechanical, chemical and flame retardant properties of neat polyblends show the improvement in the properties over single component systems. Incorporation of organo modified nanoparticles in the filler form gives further enhancement in these properties with a special mention of tensile modulus along with flame retardancy of the casted films and scratch hardness of the coatings. |
EXPERIMENTAL |
II.1. Materials |
| The reagents Bisphenol A (BA), phenyl phosphonic dichloride (PPDC), toluene diisocyanate (TDI), isophorone diisocyanate (IPDI), hexamethylene diisocyanate (HMDI) and diethylenetriamine (DETA) were procured from Aldrich and were used without further purification. The epoxy resin diglycidyl ether of bisphenol-A (DGEBA) is of commercial grade having epoxy equivalent weight of 190 gm/equiv. and was obtained from Atul Ltd. (Gujarat). Solvents such as methyl ethyl ketone (MEK), acetone (AC), methanol (MeOH) and xylene (XL) were used after distillation. The montmorillonite clay (Na-MMT) and organo modifier, hexadecyl trimethyl ammonium bromide (HDTMAB) were obtained from Sigma-Aldrich, USA. A teflon sheet of 2.5 mm thickness was punched to the required size (90 × 50 × 2.0 mm) and used for casting of films. Mild steel panels (150 x 100 x 1.25 mm) were used as substrates in all the coating applications. |
II.2. Synthesis of monomer: Bisphenol-A bis (hydroxy phenyl phosphonate) (BABHPP) |
| The monomer bisphenol-A bis (hydroxy phenyl phosphonate) was synthesized [9] by reacting bisphenol-A(1 mole) with phenyl phosphonic dichloride (2 moles) in a round bottom flask equipped with reflux condenser at a temperature of 140 °C in xylene solvent with constant stirring for 10 hours. A calculated amount of water was then added and the content was further heated at 60 °C for 4 hours with constant stirring. The crude product was washed with hot water and after filtration white solid product was obtained (Scheme. 1). |
II.3. Synthesis of polyurethanes |
| The monomer (BABHPP, 1 mole) and MEK solvent (50 wt. % of BABHPP) were taken in a 500 ml three necked flask attached with a reflux condenser, dropping funnel and thermometer. TDI (3.2 mole) was added in a dropwise manner over a period of 1 hour with constant stirring at 60 OC. Heating was continued for further 2 hours to obtain NCO terminated polyurethanes (PU-1). Pure product was obtained after vacuum distillation (Scheme. 1). Other polyurethanes PU-2 and PU-3 were also synthesized following the above method by reacting the monomer, BABHPP with the diisocyanates, IPDI and HMDI respectively [10]. |
II.4. Characterization of monomer and polyurethanes |
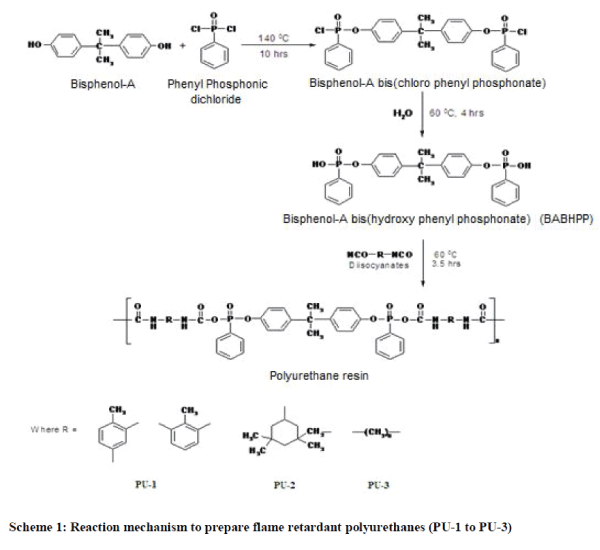
| Thin layer chromatography (TLC) was used to confirm the purity of the product. Free isocyanate content [11] of neat polyurethanes (PU-1, PU-2 and PU-3) was in the range of 1.5 to 1.9%. Number of hydroxyl group [11] of the monomer BABHPP was a 1.89 hydroxyl groups per mole of BABHPP. Intrinsic viscosity of the polymers are 0.079, 0.081 and 0.084 dl/gm as measured using ubbelohde suspended level viscometer in MEK solvent. Number average molecular weight of the polyurethanes was determined by gel permeation chromatographic (GPC) technique in Perkin Elmer USA Model Series 200. All these data are given in Table 1. Elemental analysis of polymers was carried out in Perkin Elmer USA Model 2400 series II. The infra-red spectra of the monomer and polymers were recorded in KBr pallet on Perkin Elmer USA Model Spectrum GX, FT-IR spectrophotometer (Fig.1.). |
II.5. Film casting of Epoxy/Polyurethane Blends |
| Required amount of diglycidyl ether of bisphenol-A (DGEBA) resin and curing agent diethylene triamine (DETA, 10 % Wt./Wt.) were mixed thoroughly in MEK solvent for 15 min. using mechanical stirrer to form a homogeneous solution of epoxy resin. The solution of polyurethane in MEK was then added thoroughly and carefully (to inhibit bubble formation) with continuous mechanical stirring in the epoxy solution to obtain a mixture of two component systems. The mixture was then poured on the prepared site of the mould. The set was kept at room temperature for 6 hours and post curing was carried out by placing the mould in an oven for 2 hours at 70 °C. |
 |
II.5. Film casting of Epoxy/Polyurethane Blends |
| Required amount of diglycidyl ether of bisphenol-A (DGEBA) resin and curing agent diethylene triamine (DETA, 10 % Wt./Wt.) were mixed thoroughly in MEK solvent for 15 min. using mechanical stirrer to form a homogeneous solution of epoxy resin. The solution of polyurethane in MEK was then added thoroughly and carefully (to inhibit bubble formation) with continuous mechanical stirring in the epoxy solution to obtain a mixture of two component systems. The mixture was then poured on the prepared site of the mould. The set was kept at room temperature for 6 hours and post curing was carried out by placing the mould in an oven for 2 hours at 70 °C. |
II.6. Preparation of epoxy/polyurethane clay nanocomposites |
| A homogeneous solution of DGEBA resin in MEK solvent (40 wt. % of resin) was prepared with constant mechanical stirring. Organomodified clay (o-MMT) was prepared from montmorillonite clay and hexadecyl trimethyl ammonium bromide using a reported method [12]. The o-MMT (2% Wt./Wt. of DGEBA resin) was added into the above solution with vigorous stirring for 4 hours at 60 °C to disperse o-MMT in DGEBA resin[12]. The curing agent DETA (10 % Wt./Wt.) was added thoroughly with constant stirring for 15 min. Solution of polyurethane in MEK was added to the above mixture in portions over a period of 25 min with continuous mechanical stirring. The mixture was degassed under vacuum for few minutes and then poured into a preheated mould. The filled mould was heated at 70 °C for 4 hours for the complete curing followed by post curing at 80 °C for 1 hour. The ratio of epoxy to polyurethanes was kept as 70: 30 for nanocomposites. |
 |
II.7. Testing of the polyblends and nanocomposites |
| The thickness of the films was in the range of 1.91 to 2.01 mm as measured using micrometer screw. Thermal analysis was carried out using thermogravimetric analyzer, Perkin Elmer USA Model Pyris-1, at a heating rate of 10 °C/min in air (Fig. 2 to Fig. 4). The flammability tests, limiting oxygen index (LOI) and underwriter’s laboratory test (UL-94) of the films were carried out according to the standards ASTM D 2863 and ASTM D3801 respectively. Shore D hardness of the films were measured using TSE testing machine according to ASTM D 2240. Tensile strength and elongation at break were determined using Universal Testing Machine Simadzu, Japan Autograph AG 100 KNG according to ASTM D638 type V with the crosshead speed of 20 mm/sec [13]. Izod impact strength of the films was measured using ASTM D256 method. The results are tabulated in Table 4 and Table 5. |
II.8. Preparation of coatings |
| The formulations for coatings were prepared by mixing DGEBA resin, phosphorous containing polyurethanes (PU-1 to PU-3) in different proportions and diethylene triamine (DETA) in methyl ethyl ketone (MEK) solvent. The coating formulations were applied on cleaned mild steel panels using film applicator to obtain uniform thickness of about 100 μm. The coated panels were kept at room temperature for specific time period as given in Table 2 for complete setting. To find out the effect of nanoparticles on coating properties, organo modified clay particles were added in the coating formulations in 2 wt. % amount of the ratio of 70 : 30, mixed thoroughly for half an hour using magnetic stirrer, degassed and applied on mild steel panels as described above. The coating performances of these systems are also reported in Table2. |
II.9. Characterization of coatings |
| The strength of adhesion to the coated panels and flexibility of the polyurethanes and blends were determined respectively by crosshatch and mandrel bend test methods ASTM D3359 and ASTM D522 standards [12].Scratch hardness and pencil hardness were measured according to the methods ASTM D2197 and ASTM D3363 respectively [12]. |
 |
RESULTS AND DISCUSSION |
| Elemental analysis data of polyurethanes for carbon, hydrogen, and nitrogen was in general agreement with the calculated values and are given in Table 1. Number average molecular weight (Table 1) determined from GPC data are 3557, 3812 and 2825 for PU-1, PU-2 and PU-3 respectively. |
| The IR spectrum of monomer (Fig.1.) shows broad absorption at 3348 cm-1 due to OH stretching frequency. The bands at 1041 cm-1 and 1132 cm-1 are due to P-O-C and P=O stretching frequency respectively. The band at 1438 cm-1 is due to C=C stretching frequency of aromatic ring. |
| IR-spectra of polyurethanes (PU-1 to PU-3) in Fig.1 show the broad bands around 3432-3284 cm-1 which attribute to >N-H bond. The bands around 1599-1639 cm-1 are due to –NHCOO- groups. The absorption bands around 2262-2272 cm-1 are due to free NCO groups in polyurethanes. The bands around 1058-1068 cm-1 and 1172-1199 cm-1 are due to PO- C and P=O stretching frequencies respectively. |
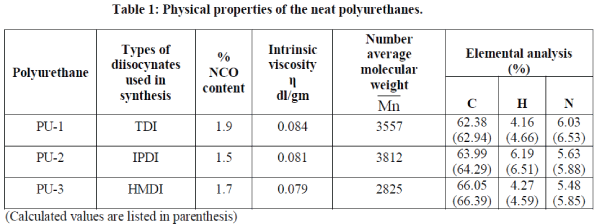
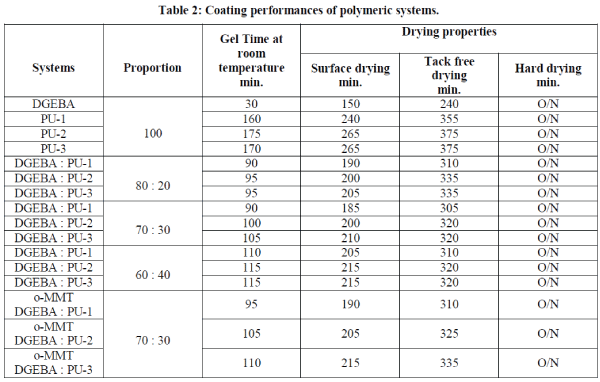
| TGA of polyurethanes (Fig.2.) show 10% weight loss around 230-235 0C temperature while the char values are in the range of 38.5-42.6 % (Table 3). The polyblends of DGEBA and BABHPP based polyurethanes in the ratio of 70: 30 (DGEBA:PU) show the remarkable increase in the stability.10% weight loss occurs around 275-290 0C (Fig. 3.). Incorporation of epoxy resin, increase the thermal stability of the polyblends. The char value decreases by 17.2-22.1% (Table 3). Presence of DGEBA is responsible for this [16, 17]. The integral procedure decomposition temperature (IPDT) values calculated from the respective thermograms using Doyle’s method [14] and the value of activation energy calculated by Broido’s method [15] are listed in this Table 2. TG thermograms (Fig.3& Fig.4) show that nanocomposites have high thermal stability compare to that of the polyblends. The temperature at 10% weight loss is increased by 15-45 °C while the char value (at 600°C) is increased by 3-4% [18]. All these data are listed in Table 3. |
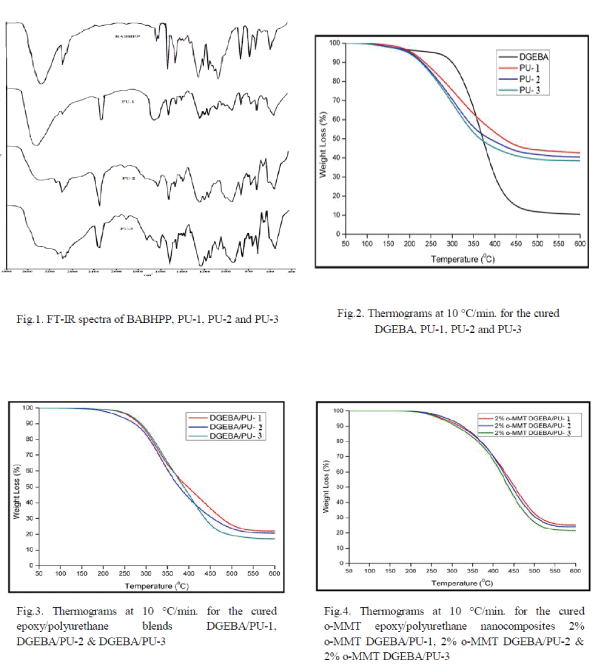 |
Table 3: Temperature characteristics and char values from thermal decomposition of the neat resin systems and their blends from TGA analysis. |
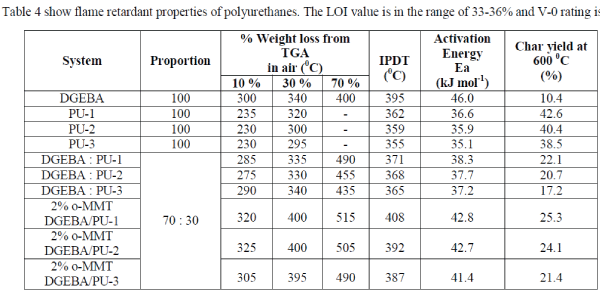 |
| obtained for the entire sample in UL-94 test. The polyblends of these polyurethanes with DGEBA show the improvement in LOI values compare to LOI value of neat DGEBA. The LOI value of the polyblends of ratios 80 : 20, 70 : 30 and 60 : 40 are in the range of 26-27%, 28-30% and 31-32% respectively. LOI value of polyblends increases as the proportion of polyurethanes increases in the polyblends[10]. The nanocomposites based polyblends of DGEBA/BABHPP based polyurethanes show the LOI values in the range of 30-33%. LOI values of nanocomposites are not very different than that of their respective polyblends. The LOI values of the nanocomposites based on TDI is than that of the IPDI and HMDI based nanocomposites which may be due to the aromatic nature of TDI. |
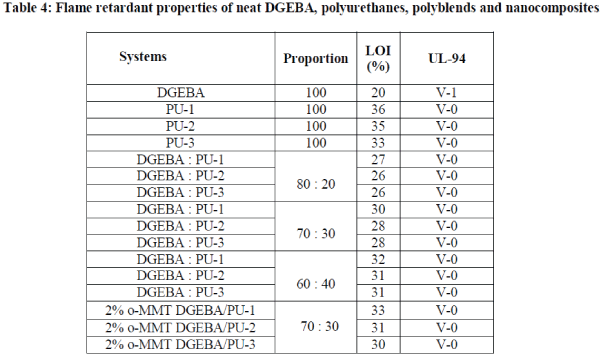 |
| The tensile strength and elongation of the polyblends (Table 5) are in the range of 65.3-49.5% and 9.2-14.5% respectively. Tensile strength of nanocomposites is increased where as elongation and impact resistance are decreased with the addition of o-MMT clay in the blends of epoxy/polyurethane [19]. Due to the flexible nature of aliphatic groups in HMDI based systems, elongation is more and a lower value of tensile strength is observed. Izod impact strength of the polyblends and nanocomposites are 8.3 to 9.4 kg-cm/cm and 7.5 to 8.1 kg-cm/cm respectively. TDI based systems have lower impact resistance than the IPDI and HMDI based systems. HMDI based systems have highest impact resistance due to the presence of aliphatic chain in the backbone chain of polymer. |
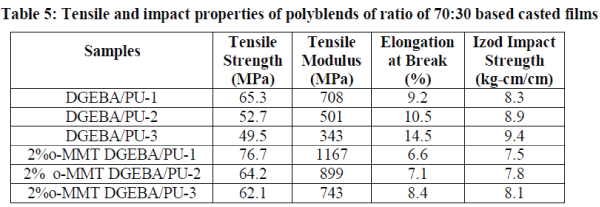 |
| Drying properties of coated panels were given in Table 2. The pot life of DGEBA is only 30 minutes while the drying properties are higher than the pure polyurethane systems. Addition of polyurethanes into the epoxy resin increases the pot life as well as drying time of the systems compare to neat DGEBA. Due to the higher reactivity of TDI the systems based on TDI show shorter pot life and drying time than the systems based on IPDI and HMDI. Addition of nano filler into the polymer blends does not affect much the pot life and drying properties of the systems. |
 |
| Mechanical properties of the coated panels were found out and the data are listed in Table 6. Lowest value of scratch hardness and pencil hardness were observed for all the neat polyurethane based coatings in the series. Their poor film forming properties is responsible for this. When these polymers were incorporated into epoxy resin in different proportions both the scratch and pencil hardness properties have been improved. Further improvements in these properties have been observed by the addition of o-MMT. It has also seen that all the systems have good adhesion properties. Here again the neat polyurethanes response faster than the other coating systems. |
CONCLUSIONS |
| Phosphorous containing polyurethanes have been synthesized and used for preparing polymer blends with conventional DGEBA resin which show improved properties over the neat polyurethanes. Nanocomposites of polyblends with organomodified clay, show improvement in tensile modulus over the neat polyblends, while in these systems decrease in elongation is observed with the incorporation of organo modified nano clay. The formulations when used in coating applications show very good enhancement in scratch resistance properties over the neat polyblends. |
ACKNOWLEDGMENTS |
| The financial assistance (F. No. 32-99/2006 (SR)) from University Grants Commission (UGC), New Delhi, India is gratefully acknowledged. Appreciation is expressed to Sophisticated Instrumentation Center for Applied Research and Testing (SICART), Charutar Vidya Mandal, Vallabh Vidyanagar, for the analysis of the samples. |
References |
|