e-ISSN: 2319-9849
e-ISSN: 2319-9849
Kovalyshyn Y1, Konovska M1, Krupak A1, Milanese C2, Saldan I1,2* and Reshetnyak O1
1Department of Physical and Colloid Chemistry, Ivan Franko National University of Lviv, 6 Kyryla and Mefodia, 79005 Lviv, Ukraine
2Pavia H2 Lab, CSGI and Chemistry Department, Physical Chemistry Section,University of Pavia, 16 Viale Taramelli, 27100 Pavia, Italy
Received date: 03/09/2016; Accepted date: 23/09/2016; Published date: 30/09/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
Polyaniline composites with multi-walled carbon nanotubes modified by benzene-, amino- and aminobenzene groups were chemically synthesized. FTIR spectroscopy suggests parallel orientation of polyaniline benzene rings to the surface of carbon nanotubes while aminobenzene groups previously grafted to the nanotubes surface participate in oxidative condensation process for aniline and hence cause growth of polymer chain nonparallel in respect to the surface. It is suggested that structural similarity between aminobenzene and aniline facilitates the condensation reaction and results in a higher number of chemical bonds between polyaniline and carbon nanotubes. Surface modification by amino- or aminobenzene groups promotes higher specific electric conductivity and electrochemical capacitance while the modification by benzene groups leads to a steep decrease in the capacitance and one-order of magnitude lower values of electric conductivity.
Polymer composites, Surface modification, FTIR, Cyclic voltammetry
Among the great variety of C-based materials, special attention is paid to electroactive composites based on conjugated polymers with carbon nanotubes (CNTs). Polyaniline (PAni) is in a special account among the polymers because of its simplicity in synthesis and application [1]. PAni is alinear polymer with alternation of quinone- (-N=Q=N–) and benzenediamine (–NH–B–NH–) fragments where Q and B is quinone- and benzene ring, respectively [2]. The polymer structure is emeraldine salt in protonic stage (acid doped) and emeraldine base when deprotonated [3]:
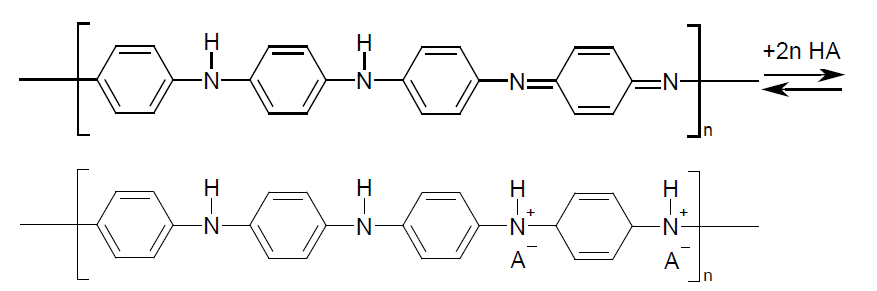
Reversibility of redox reactions between the different forms of PAni and facility of doping-dedoping allows the regulation of the oxidation state of nitrogen and hence of the number of charged groups in the polymer. In protonic state PAni shows a good ionic and electron conductivity, hence high electrochemical capacitance that makes it a promising polymer for effective electroactive composite materials [4]. It is noticeable that the first polymer electrode for chemical power source was made of PAni [5,6].
For the electroactive composites the CNTs might be very suitable since they possess unique physical and chemical properties (high thermo- and mechanical stability, electric conductivity, etc.) However, a huge scientific experience confirms the problem of high dispersity and homogeneity of CNTs in a polymer matrix that is a crucial factor of their effective usage in the composites. One of the solutions might be surface modification of CNTs. On the one hand, it can be non-covalent immobilization of chemical compounds due to van der Waals forces, electrostatic or π-electron interaction. On the other hand, it can be functional groups covalently bonded to the outer wall of CNTs [7], where the modifier could be benzenediazonium tetrafluoroborate [8], since its cation can be easy reduced at the place of diazo group:
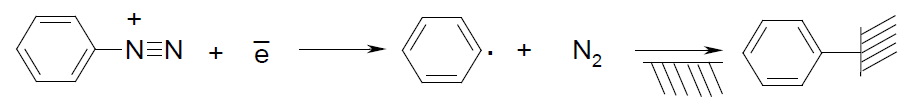
As the result, obtained benzene radicals can be chemically grafted to the support while molecular nitrogen releases. In practice, the highly reactive radicals can cover CNTs surface up to a complete benzene layer [9].
In order to promote interaction between CNTs and polymer matrix in the composites, chemical grafting of aminobenzene groups to the CNTs surface is proposed in the present work. It was suggested that during the composites synthesis these functional groups can react with aniline by oxidative condensation reaction to produce PAni chemically bonded to the CNTs. Fourier transform infrared (FTIR) spectroscopy was used to analyze the proposed surface modification of CNTs. Specific electric conductivity and electrochemical capacitance were calculated based on experimental data. The relationship between surface modification of CNTs and properties of the obtained composites was discussed in details.
Aniline (Sigma-Aldrich, 99%) was distilled in purified argon before using. The 4-nitroaniline (≥ 99%), cellulose (medium fibers), potassium chloride (≥ 99%), sodium persulfate (≥ 98%), sodium hypophosphite monohydrate (≥ 99%) and acetone (≥ 99.8%) were delivered from Sigma-Aldrich and used without additional purification. The hydrochloric, nitric, sulfuric and tetrafluoroboric acids were received from Alfa Aesar as their aqueous solutions with concentrations of 33, 65, 92 and 48%, respectively. Multi-walled CNTs (MWCNT; 1000-50 000 nm in length; outer diameter of 10-50 nm; specific surface of 200-400 m2/g) were synthesized by catalytic chemical vapor deposition using the procedure developed at the Institute of Surface Chemistry of NANU [10].
Benzenediazonium tetrafluoroborate (BT) and 4-nitrobenzenediazonium tetrafluoroborate (NT) were synthesized according to the method proposed in group of Sakakura [11]. Synthesis of nitrocellulose was performed with regards to the full description made by Dombrovski et al. [12]. Obtained products were rinsed by distilled water, dried in air and grinded by mortar.
Surface Modification of MWCNTs
As received MWCNTs were modified by BT (MWCNT-1) followed by treatment in nitrating mixture (MWCNT-2). The NT-modified MWCNTs was called MWCNT-3. Abbreviation of MWCNT-0 was assigned to as received MWCNTs.
The MWCNT-1 and MWCNT-3 were chemically synthesized when solution of sodium hypophosphite (50 wt.%) was stirred with BT or NT (50 mol × 10-3), correspondently, and then MWCNT-0 (0.05 g) were added [8]. Total mixture was made within 30 min and filtrated with distilled water and acetonitrile to remove residual sodium hypophosphite and the used diazo salts (BT, NT). Additionally, the prepared MWCNT-1 was dried in furnace at the pressure of 0.02 bar and constant temperature of 25 ºС during 20 h. The MWCNT-2 was produced by a reaction between MWCNT-1 (1.5 g) and equimolar mixture of nitric and sulfuric acids (6 ml) during 3 min of stirring [13]. The obtained solution was filtrated with distilled water and dried as MWCNT-1.
Chemical reduction of NO2 groups in MWCNT-2 and MWCNT-3 (1.23 g of each) was carried out by way of solving of metallic zinc (2 g) in hydrochloric acid (8.5 ml) [14]. The obtained mixture was fractionally distilled during ∼5 h and then filtered with distilled water to remove residual hydrochloric acid. The treated MWCNT-2 and MWCNT-3 were dried in furnace as MWCNT-1. Sample names and description of their surface modification is summarized in Table 1.
| Name | Surface modification of MWCNT |
|---|---|
| MWCNT-0 | As received |
| MWCNT-1 | Modification by benzenediazonium tetrafluoroborate |
| MWCNT-2 | Modification by benzenediazonium tetrafluoroborate, treatment in nitratingmixture and chemical reduction of nitro groups |
| MWCNT-3 | Modification by the 4-nitrobenzenediazonium tetrafluoroborate and chemical reduction of nitro groups |
Table 1: Description of the used surface modification of MWCNT.
Synthesis of PAni Composites with MWCNTs
Chemical synthesis of PAni composites was made in the mixture of aniline (0.1 M) and hydrochloric acid (1.0 M) [15]. Addition of sodium persulfate was followed by introduction of MWCNTs as the final ingredient of the reactive mixture. The total mixture was mechanically mixed during 30 min and kept at the room temperature during a day. Eventually, the mixture was filtered with distilled water and dried in the furnace at the pressure of 0.02 bar and constant temperature of 70ºС during 12 h. According to the procedure, the PAni composites with 1-20 wt% of MWCNTs (MWCNTs=MWCNT-0, MWCNT-1, MWCNT-2, MWCNT-3) were synthesized.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) spectroscopy was used to detect the functional groups in wave number range of 4000-700 cm-1. The FTIR spectra were collected in attenuated total reflection in the transmittance mode using a NICOLET iS10 instrument, THERMO SCIENTIFIC. Data acquisition was taken in 256 scans with resolution of 4 and spacing of 0.482 cm-1. For every sample a background (FTIR spectrum for empty sample holder) was measured and subtracted from the FTIR spectrum for the sample obtained in the same conditions.
Specific Electric Conductivity
Electrical resistance R was measured using an ohmmeter with four-terminal sensing. Prepared composites were grinded in mortar and pressed in tablets with the radius r and thickness h to calculate the volume and hence the density. Using the measured values, specific electric conductivity χ was calculated as:
Χ=h/(πr2R) (1)
Specific Electrochemical Capacitance
Electrochemical capacitance of the prepared composites was estimated by cyclic voltammetry (CV). The composite powders (0.002 g) were placed on the working electrode and impregnated with a 1.0 wt% solution (10 × 10-6 l) of nitrocellulose in acetone followed by drying in air at room temperature. The CV was carried out in a three-electrode cell using PR-8 programmed potentiostate. A rotated graphite disc was encapsulated in fluoroplastic cartridge case in diameter of ~3 mm and used as a working electrode. A platinum plate (S=1.0×10-3 m2) acted as a counter electrode. All the experimental potential values were referred to Ag/AgCl in saturated KCl solution as silver chloride (SCE) reference electrode. Within the range of applied potential from –0.100 to 0.800 V the 10 scans were made in aqueous 1.0 М НСl at various scanning rate (5-100 mV/s). Calculation of specific electrochemical capacitance (Сs) was made according to the formula:
Сs=Imax/[(dV/dt) × (W)] (2)
where Ima, dV/dt and W is anode current at 0.600 V, scanning rate and weight of active material, respectively [16].
FTIR spectroscopic analysis
FTIR spectroscopy was applied to analyze functional groups in the MWCNTs (Figure 1) and the prepared composites (Figure 2) using reliable data base [17,18].
The spectrum of the as received MWCNTs shows mild absorption bands in the region of 1600-1400 cm-1 that correspond to vibration of С=С bonding in conjugated benzene rings (Figure 1). There are no other absorption bands responsible for any functional groups in the spectrum. Absorption bands that confirmed appearance of substituted benzene rings and NO2 groups were identified for MWCNT-3 (Figure 1). Absorption bands at 1506 cm-1 and 1338 cm-1 are correspondently asymmetric and symmetric covalent vibrations of NO2 groups. Additional confirmations of the presence of NO2 groups are signals at 848 cm-1 and 735 cm-1 with a low intensity. The absorption at 1590 cm–1 region is due to benzene rings. Appearance of NH2 groups was identified for MWCNT-3 after chemical reduction of NO2 groups (Figure 1). The spectrum shows several absorption bands that correspond to the presence of NH2: wide and intense band within the 3500-3300 cm-1 range is due to valence vibrations of NHbond; the 1621 cm-1 signal is the plane ring vibrations for deformation of NH2 groups; and the one centered at 1273 cm-1 is the characteristic band of primary aromatic amines. Vibrations of benzene rings are evident from the bands within the 1600-1400 cm-1 rangeIt is noticeable that absorption at 2979 cm-1 and the more intense one at 2899 cm-1 correspond to valence vibrations of СН– bond. This fact suggests chemical reduction of unsaturated bonds between carbon atoms to produce СН2– and СН3– bonds at various defects points of MWCNTs.
Spectrum for PAni shows typical absorption bands of emeraldine salt (Figure 2). The vibrations of the quinone- and benzene rings in the polymer chain are confirmed by signals at 1557 cm-1 and 1470 cm-1, respectively. The absorption at 1435 cm-1 is due to –С=С– bond [18-20] while the one at 1027 cm-1 is deformation vibrations in benzene rings [21]. Small shoulder of this band is due to vibration fluctuations of protonated amino groups (1100 cm-1). Absorption bands at 1285 cm-1 can be attributed to CN– vibrations in secondary amines while at 1234 cm-1 the vibration of CN●+ in the polaron lattice of PAni is active. The wide band of absorption in 2200-1630 cm-1 region is typical for conjugated double bonds (С=С, С=N and C=N+) in polycation radical of emeraldine salt [17] that specifies the electron conductive state of PAni [22]. Since spectroscopic changes in this region indicate differences in the conjugated system, another charge transfer process is suggested. The low intensity band at 875 cm-1 caused by out of plane ring vibrations for deformation of СН– bond of benzene ring. Spectrum for PAni composite with 20 wt% of MWCNT-0 (Figure 2) is similar to that of pure PAni (Figure 2) although some peculiarities might be specified. There is an increasing relative intensity of absorption bands at 1435 cm-1 that is attributed to –С=С– bond in benzene rings and at 1227 cm-1 for the one due to CN●+. It suggests a higher number of charged groups in PAni. In general, the absorption in 2200-1630 cm-1 region is similar to that of pure PAni, meaning no influence of the added MWCNT-0 on the structure of whole conjugated system and charge transfer process in PAni. Moreover, decrease in intensity of absorption bands at 875 cm-1 means reduction of out of plane ring vibrations for deformation of СН– bond in benzene rings. The FTIR spectrum for the composite could be explained by adsorption of molecular aniline on the MWCNT-0 surface because of applied synthesis. Obviously, synthesized PAni must be oriented to MWCNT-0 surface in a special way. It is suggested that benzene rings of the polymer mainly parallel oriented to the surface, otherwise out of plane ring vibrations for deformation of СН– bond would be suppressed. In order to check this hypothesis another procedure of synthesis for PAni composite with 20 wt% of MWCNT-0 was made when the MWCNTs were added to the reactive mixture simultaneously with aniline and sodium persulfate solutions. FTIR spectrum of the composite prepared in that way is shown in Figure 2 and used MWCNTs called as MWCNT-0-A. Relative intensity decrease of the band responsible for CN●+ at 1240 cm-1 is observed compared to that of pure PAni (Figure 2). This suggests a lower number of the charged groups. Absorption in 2200-1630 cm-1 region is changing regards to that of pure PAni, and splitting of the absorption band takes place. A sharp absorption band in 1700-1600 cm-1 region is visible for the composite (Figure 2). Thus, addition of MWCNT-0-A effects the structure of conjugated system and charge transfer process in PAni. This effect might be explained by a new chemical bonding between PAni and MWCNT-0-A. Moreover, a confirmation of this might be the absorption band at 875 cm-1 that is the same as that of pure PAni. That is, no such obstacles at out of plane ring vibrations for deformation of СН– bond of benzene ring, as in case of PAni-MWCNT-0 composite. Spectroscopic analysis of another synthesized PAni composite with MWCNT-0-A suggests that aniline has no time to be adsorbed on the MWCNT-0-A surface. During this synthesis procedure the interaction between intermediate ion-radicals and MWCNT-0-A produces СN– bonds between nitrogen atom of aniline and carbon atoms of carbon nanotube. As a result, the growth of polymer chains occurs non-parallel respect to the surface. Indeed, spectrum for PAni composite with 20 wt% of MWCNT-3 after chemical reduction of NO2 groups (Figure 2) is almost identical to that of PAni composite with MWCNT-0-A (Figure 2). A small increase in intensity of the band dedicated to vibration of CN●+at 1234 cm-1 also suggests a higher number of the charged groups. In addition, splitting of absorption band at 2200-1630 cm-1 region (typical for conjugated double bonds) indicates some changes in charge transfer process similar as for composite of PAni with MWCNT-0-A. These facts confirm the hypothesis that aminobenzene groups grafted to MWCNT-3 surface participate in oxidative condensation process for aniline and hence cause growth of polymer chains non-parallel respect to the surface.
Specific Electric Conductivity of PAni Composites with MWCNTs
Specific electric conductivity was calculated for all prepared PAni composites with MWCNTs (Figure 3). Values of the specific electric conductivity for MWCNT-1 are a bit higher compared to that of MWCNT-0 (inset in Figure 3) though similar at 1-5 wt% of the MWCNTs. The percolation threshold can be achieved at approximately 7-10 wt% of MWCNTs for both composites and confirms physical and chemical interactions between PAni and MWCNTs. Further gradual increase in the specific conductivity could be explained by a higher content of MWCNTs as the component with relatively higher conductivity. Most probably, better contact between composite components is in case of MWCNT-1 that facilitates electron transfer between macromolecular chains and MWCNT-1, and hence results in higher electric conductivity. A paraboloid dependence of specific electric conductivity on the MWCNTs content is found for both chemically reduced MWCNT-2 and MWCNT-3 (Figure 3). The specific electric conductivity steeply increases in the frame of 1-3 wt% of the MWCNTs and then starts to be stabilized. A low value of the percolation threshold (∼1-2 wt% of the MWCNTs) for both composites suggests stronger interaction between the composite components. It is noticeable that amino- and aminobenzene groups, correspondently on the MWCNT-2 and MWCNT-3 surface, chemically reacted with aniline in the oxidative condensation process to produce PAni grafted to the MWCNTs. The highest values of specific electric conductivity were obtained for PAni composite with chemically reduced MWCNT-3. It is reasonable to think that structural similarity between aminobenzene group and aniline facilitates the oxidative condensation process and hence appearance of a higher number of chemical bonds between PAni and MWCNT-3.
In order to check this hypothesis, the dependence of apparent density (as the ratio of weight to the volume of the porous composite) on the chemically reduced MWCNT-3 content was analyzed for the pressed (250 kg/cm2) PAni composites (Figure 4). The minimum of apparent density at approximately 3 wt% of the MWCNT-3 was experimentally observed. It is suggested that at low MWCNT-3 content every MWCNT-3 can form a very high number of chemical bonds directly with PAni macromolecules and local places without bonding can produce pores. At the higher MWCNT-3 content the number of chemical bonds between PAni and MWCNT-3 decreases because of MWCNT-3 agglomeration. The oxidative condensation process takes place in the liquid phase homogeneously and produces oligomers that eventually form dispersed solid polymer. Effective area of interphase surface is quite high hence the rate of PAni deposition, related to the surface is low, that causing formation of well-packed PAni macromolecules. According to the proposed hypothesis, the MWCNT-3 agglomeration starts to be at values higher than 3 wt% of the MWCNTs (Figure 4). The obtained dependence of the apparent density is not in agreement with that of specific electric conductivity that hints on a special ordering of MWCNTs (not a spontaneous MWCNTs agglomeration) to get high conductivity.
Electrochemical Capacitance of PAni Composites with MWCNTs
For the prepared PAni composites, CV at various scanning rates was measured (Figure 5). The electrical current increases during gradual increase of the scanning rate. For the PAni composite with MWCNT-0 and MWCNT-1, the current maximum, that corresponds to redox processes in PAni, is slightly visible (Figure 5) while it is totally absent for those with MWCNT-2 and MWCNT-3 (Figure 5). Experimental values of the electrical current for the composite with MWCNT-2 and MWCNT-3 are one order of magnitude higher compared to those with MWCNT-0 and MWCNT-1. This suggests their higher contribution of the charge current of double electric layer to the measured electrical current and it is in agreement with higher values of specific surface area of the composites.
The dependence of CV current at 0.600 V during anodic process on the square root of the potential sweep rate were analyzed for the prepared PAni composites (Figure 6). For planar diffusion-controlled electrochemical process the peak of current (Ip) is estimated by Randles-Sevcik equation:
Ip=k2/3AD1/2Cυ1/2 (3)
where k, А, D, C and υ are the Randles-Sevcik constant, electrode surface area, diffusion coefficient, volume concentration of the active material and potential sweep rate, respectively. Indeed, for the PAni composite with MWCNT-0 and MWCNT-1 the peak current is proportional to the square root of the sweep rate as predicted by the Equation 3. In case of MWCNT-2 and MWCNT-3, their composites show paraboloid dependences and their current values are one order of magnitude higher in comparison to those with MWCNT-0 and MWCNT-1. This could be explained by a more developed effective surface area (A value in Equation 3). Obviously, at higher potential sweep rate, diffusion is not enough to guarantee appearance of active material close to the working electrode. It leads to the situation when not the whole electrode surface is involved in the electrochemical process. In other words, some parts of the surface can be “switched off” during the process.
Based on experimental current values at 0.600 V during anodic process, specific electrochemical capacitance was calculated (Figure 7). For the PAni composite with MWCNT-0 and MWCNT-1 (Figure 7) within range of 0-2 wt% of MWCNTs content calculated capacitance decreases. It suggests low chemical interaction between the MWCNTs and PAni hence low electrical contact of PAni to electrode surface through the MWCNTs. Moreover, for the composite with MWCNT-1 within the range of 5-15 wt% of the MWCNTs calculated capacitance decreases steeply. It suggests faster decrease of Faraday current due to redox processes in PAni in comparison with the increase in the charge current of a double electric layer. The maximal values of calculated capacitance for the composite with MWCNT-2 and MWCNT-3 are observed with wt% of MWCNTs amount higher than 2 wt% (Figure 7). As it was mentioned above, better physical and chemical interactions between PAni and MWCNTs was suggested for MWCNT-2 and MWCNT-3. Moreover, for the composite with MWCNT-2 high value of the calculated capacitance was found within wide range of 2-20 wt% of MWCNTs content (Figure 7). This could be explained by additional activation of MWCNTs during their treatment in nitrating mixture. It is suggested that the treated MWCNT-2 surface can be covered by additional (OH– and COOH–) groups or slightly defected, that leading to additional increase in the specific surface area.
The PAni composites with as received MWCNTs (MWCNT-0), MWCNTs modified by benzene groups (MWCNT-1), by amino- (MWCNT-2) and aminobenzene groups (MWCNT-3) were chemically synthesized. FTIR spectroscopy confirms surface modification of MWCNTs by 4-nitrobenzenediazonium tetrafluoroborate that results in chemically grafted 4-nitrobenzene groups and, after following a chemical reduction, to aminobenzene groups. The spectrum for PAni shows typical absorption bands of emeraldine salt. FTIR spectrum for the PAni composite with MWCNT-0 suggests that benzene rings of the polymer are oriented mainly parallel to the MWCNT-0 surface. For the PAni composite with MWCNT-3, aminobenzene groups previously chemically grafted to the MWCNTs surface participate in oxidative condensation process for aniline and hence cause growth of polymer chain non-parallel with respect to the surface.
The highest values of specific electric conductivity were obtained for PAni composite with MWCNT-3. Most probably, structural similarity between aminobenzene group and aniline facilitates the oxidative condensation process and hence the higher number of chemical bonds between PAni and the MWCNTs. It is suggested that agglomeration of MWCNT-3 starts at MWCNTs content higher that 3 wt.%. The experimentally obtained dependence of apparent density is not in agreement with that of specific electric conductivity that points to a special ordering of MWCNTs to get high conductivity.
Experimental values of the electrical current for the composite with MWCNT-2 and MWCNT-3 are one order of magnitude higher compared to those of MWCNT-0 and MWCNT-1. This suggests their higher contribution of the charge current of the double electric layer to the measured electrical current and it is in agreement with higher values of specific surface area. Surface modification of MWCNTs by amino- or aminobenzene groups results in high values of specific electrochemical capacitance of their composites beginning from 2 wt% of MWCNTs while the composite with MWCNTs modified by benzene groups shows a steep decrease in the calculated capacitance within range of 5-15 wt% of MWCNTs.
The research work was financially supported by Cariplo 2013-0592 project obtained by Italian Cariplo Foundation and ХФ- 149Ф project of the Ministry of Education and Science of Ukraine.