e-ISSN: 2319-9849
e-ISSN: 2319-9849
Maxmilla M1*, Changamu E2, Andala D3
1 Department of Science Technology and Engineering, Kibabii University, Kenya
2 Department of Chemistry, Kenyatta University, Kenya.
3 Department of Chemistry, Multi Media University, Kenya
Received date: 14/03/2021; Accepted date: 05/04/2021; Published date: 12/04/2021
Visit for more related articles at Research & Reviews: Journal of Chemistry
Phosphorus obtained from finite rock phosphate is one of the essential elements for food production and modern agriculture. Therefore, for sustainability of food supply and development in agriculture, management of phosphorus is key. Animal bones have a high concentration of phosphate that can be harvested and used in fertilizer production but they take many years to decompose and release the phosphate. This study set out to prepare bone phosphate enriched phosphoric acid from the otherwise discarded animal bones. Animal bones (predominantly cattle bones) were collected, washed, dried and crashed to smaller particles using a hammer after which they were ground in a mill. The ground bones were extracted with 0.275 M H3PO4 to give a 4.58 M bone phosphate-enriched phosphoric acid solution. Nitrogen was extracted from air by passing the air over heated copper filings and reacted with lithium to form lithium nitride. The lithium nitride was later hydrolysed to form ammonia which was reacted with the bonephosphate enriched phosphoric acid to generate the diammonium phosphate (NH4)2HPO4) fertilizer. The percent composition of nitrogen (Kjeldahl) in the diammonium phosphate was found to be 17.14% N while that of the phosphate was found to be 44.58% P2O5. The efficacy of diammonium phosphate in the growing of tomatoes in a greenhouse was determined with the commercially obtained diammonium phosphate as the positive control and no fertilizer as the negative control. Growth parameters of the tomatoes including plant heights, leaf length, leaf width and root length were obtained over a period of twelve weeks. The results showed that the growth parameters recorded for tomato plants grown with synthesized fertilizer were not significantly different from those recorded for tomato plants grown with commercial fertilizer (p-values = 0.000 <0.05). Based on the results obtained, it can be concluded that the bone phosphate based fertilizer prepared in this study was as efficacious as the commercial fertilizer. This is a significant finding in that it shows that it shows that animal bones can be converted into readily available phosphatic fertilizer.
Animal bones, Enriched phosphoric acid, Diammonium phosphate Fertilizer.
Zero hunger is the United Nations’ sustainable development goal number two whose main objective is to eliminate all forms of hunger and malnutrition by 2030 [1]. This is achieved by making sure that all people (especially children) have sufficient and nutritious food throughout the year [1]. It includes promotion of agriculture that is sustainable, small-scale farmer support, equal accessibility to land, improved technologies, accessible to ready markets and development of infrastructure [2]. Phosphorus availability is one factor that is likely to stand in the way of attaining this sustainable development goal as well as its successor(s) for phosphate fertilizers. A recent review article in Contemporary Agriculture summarized the issues related phosphorus thus,
“Phosphorus is an essential nutrient for all forms of life, which means that food cannot be produced without it. As the phosphate rock (concentrated source of phosphorus) is a non-renewable and finite resource, with no substitute, without more sustainable management of phosphorus its deposits could be depleted in a rather short period. In addition, much of phosphorus eventually ends up in environment, where it causes pollution. Hence, one could say that the lack of phosphorus and its inappropriate management could be a bottleneck for a sustainable food supply and agricultural development in general. Nevertheless, unlike some other challenges that modern agriculture has to face (for example, water and energy scarcity, climate changes etc.) the problem of phosphorus limited availability and accessibility has been largely neglected until recently” [3].
Phosphorus is currently supplied through phosphate fertilizers made from finite phosphate rock and therefore it has to be managed for sustainability of adequate supply of food and development of agriculture. There is however a lot of uncertainty because high quality reserves could be depleted in the next 30–40 years. Deposits that are of low grades as a result of contamination by heavy metals such as cadmium or uranium are likely to remain [4]. Additionally, extraction and purification of these low grade deposits also result in environmental problems that result from huge amount of waste phosphogypsum contaminated by radionuclides [4].
The process of producing phosphate fertilizers from rock phosphate has continued since 1867, and an increasing number of studies suggest that global phosphate rock extraction will reach its peak in the coming decades. The remaining potential reserves are characterized by poor quality that is costly to extract [5-9]. There is, however, no clear consensus on when extraction is likely to reach peak. Cordell et al., and Cordell, estimates phosphorus to reach its peak between 50–100 years while Steen, Driver et al., and Stewart et al., reiterate that tis estimates exclude reserve bases that are not economical to mine. Cordell et al., estimates the peak to be 350 years based on current production capacity excluding increased demand for phosphorus. The scarcity of natural rock phosphates is as a result of losses during agricultural runoff, erosion, animal wastes, hence, need for a more effective approach that addresses the future of phosphorus scarcity. This includes and not limited to exploring mechanisms that minimize leakage, recovery and recycling of phosphorus from various processing chains [10].
Larsen and coworkers suggested that extraction of phosphorus from waste water is a possible solution to large dependence on fossil deposits. So far the importance of nutrient recovery from wastewater has been underestimated, but it is clear that it could significantly contribute to overcome the looming phosphorous crisis [11]. As more and more people move from rural to live in cities, food is moved from production zones to the cities and with it incorporated phosphorous which eventually ends up in municipal waste waters, slaughterhouse wastes and landfills. Recovery of phosphorus from such sources for food production should be priority going forward. Manufacture of phosphorus fertilizers from locally available organic waste products, such as human excreta, industrial organic waste by-products, animal dung, fish, ash, bones, and other slaughterhouse by-products was practiced before the discovery of chemical production [10]. In early 17 and 18th centuries, England imported bones from its neighbors and used them to supplement animal and human excreta which were being used as sources of phosphorus [10].
Animal bones which contain carbonate–hydroxyl–apatite structure are always disposed of as wastes by abattoirs in large cities and towns yet they are sources of high concentration of phosphate that can be harvested and used in fertilizer production. Bones are the most concentrated forms of organic phosphates derived from feed and feed supplements and they are used for crop production. They have the highest phosphorus concentration in water soluble fraction (505 mg/kg) as compared to processed phosphate which has 307 mg/kg [12]. The calcium and phosphorus contents (25–29% Ca and 15 -19% P) compares well the natural rocks (35 ± 2% Ca and 15 ± 1% P) [13]. Experimental results by Cayuela [14] indicate that when the soils are treated with bone meal there is an increase in carbon minerals, nitrogen, microbial biomass size and activity. Therefore, using bones in this study presents a recycling process of organic phosphorus.
Diammonium phosphate (DAP) fertilizer is a widely used phosphorus fertilizer applied to soils to address low levels of phosphorus as a result of intense agriculture [15]. It contains nitrogen which is important in promoting rapid vegetative growth and phosphorus which is a component of ATP and chlorophyll that is essential for food production. DAP is known to have good physical properties such as high solubility that makes it a popular choice in farming and use in other industries. It has other uses that include being used as a fire retardant and metal finishing [15]. It is produced from two common constituents, ammonia (from the Haber process) and phosphoric acid from phosphate rock. Industrial manufacture of phosphoric acid is done mainly by thermal and wet processes. In this work, bone phosphate enriched phosphoric acid is prepared from the otherwise discarded animal bones.
This section describes the research methods, procedures and materials used in the preparation of diammonium phosphate (DAP) fertilizer. It also describes the characterization of the diammonium phosphate synthesized in terms of percent composition of nitrogen and phosphorus as well as the efficacy studies in growing of tomatoes in a greenhouse.
Animal bone preparation
Bones were collected from Kachok Municipal dump site, Kisumu West District, Kenya. They were washed with running tap water, rinsed with de-ionized water and dried in a temperature-controlled oven (WTC binder, 150533, Germany) at 110°C for twelve hours. They were reduced in size using a hammer at Kenyatta University Physical Chemistry Research Laboratory and ground using a grinding machine at the Geology and Mines laboratories, Kenya, to enhance the rate of digestion in phosphoric acid.
Bone phosphate extraction
This was done following the procedure highlighted in the article Manufacture of phosphoric acid from hydroxyl apatite contained in the ashes of the incinerated meat–bone ashes by Zuczek [16]. The process of evaluating the lowest concentration of phosphoric acid for total dissolution of bones was carried out by varying the concentrations from 0.1 M, 0.2 M, 0.25 M, 0.275 M and 0.3 M. The concentrations were each exposed to 10 g of bones each at 75°C and agitated for two hours. The concentrates were filtered; the unreacted bones were rinsed with deionized water and dried by squeezing between two filter papers. The filtrate was reacted with 6 M H2SO4 acid to precipitate the Ca2+. It was filtered and the acid concentration in the filtrate determined by back titration. The% P in the filtrate was determined and results recorded in Table 4. From the results it was determined that the lowest concentration of phosphoric acid to completely dissolve bones was 0.275 M. Therefore, volumes of 10000 ml of 0.275 M phosphoric acid were used to dissolve 10 kg of bones in plastic containers for a period of seven days. A stirring bar was used for stirring the solutions after every twenty four hours to ensure that all the bones were dissolved. The laboratory temperatures varied between 21–25°C during this period of experiment. Ten twenty-litre plastic containers were used in dissolution of bones and replicate measurements were done for a period of two months to dissolve about 130 kg of bones and to confirm the solubility data. The resulting mixture was filtered and stored. 5.0 ml of the sample was measured and dissolved in 50 ml of deionized water each. The solution was then transferred to three 100 ml of volumetric flasks separately and diluted up to the mark with deionized water [17]. A solution of each sample (1.0 ml) was transferred to a 25 ml volumetric flask followed by 2 ml of 2.5% ammonium molybdate and 0.5 ml of 1 M sulphuric acid solutions. The mixture was shaken before adding 1.0 ml of 0.5 M hydrazine hydrate solution and the volume made up to mark with deionized water [17]. Colour developed fully when the solution was allowed to stand for 45 minutes. Absorbance was taken at a wavelength of 830 nm [17]. A calibration curve (Figure 1) for the standard phosphate solutions was used to calculate the concentration of phosphate in the enhanced phosphoric acid. The values obtained for PO43- were converted to Total phosphorus by multiplying with 0.3261 and the values were converted to P2O5 by multiplying by 2.2915.
Synthesis of Diammonium Phosphate
An average amount (156.09 g) of lithium nitride was ground in a mortar and placed in a flat bottomed flask and a separatory funnel attached to the flask. 150 ml of deionized water was added to the flat-bottomed flask through the separatory funnel to initiate hydrolysis. The ammonia gas produced passed through a delivery tube to a beaker containing 200 ml of 4.58 M H3PO4 extracted from bones as described in Section 3.10. This experiment was repeated eight times to enable use of all the lithium nitride solid that had been prepared. The beaker containing 4.58 M phosphoric acid (200 ml) was immersed in basin containing ice because the reaction was highly exothermic. The highest temperature recorded during the reaction was 95°C but the crystals formed at 51°C. The crystals formed were filtered through Whatman No 1 filter paper, air dried at room temperature for seven days and weighed. A total of 3170.60 g of the crystals formed were analysed for percentage composition of phosphorus spectrophotometrically.
Characterization of diammonium phosphate fertilizer
Determination of phosphorus in the fertilizer
The sample of synthesized fertilizer (5.0 g) was weighed out and dissolved in 50 ml of deionized water. The solution was then filtered through a Whatmann-41 filter paper and the filtrate transferred to a 100 ml of volumetric flask and diluted up to the mark with deionized water [17]. A solution of sample (1.0 ml) was transferred to a 25 ml volumetric flask followed by 2 ml of 2.5% ammonium molybdate and 0.5 ml of 1 M sulphuric acid solution [17]. The mixture was shaken before adding 1.0 ml of 0.5 M hydrazine hydrate solution and the volume made up to mark with deionized water. The solution was allowed to stand for about 45 minutes for maximum colour development [17]. Absorbance was taken at a wavelength of 830 nm. Similarly, the absorbance values for the fertilizer samples were measured and the corresponding concentrations of phosphorus were obtained using the equation from the calibration curve [17]. The procedure was replicated for the commercial fertilizer. From the experimentally determined phosphorus concentration, the percentage P2O5 in the fertilizer was calculated from the equation (1) below:
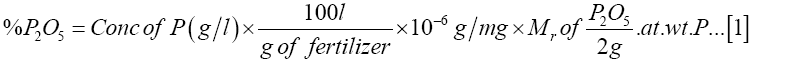
Where g. of fert = weight of fertilizer measured
Mrof P2O5 = Molecular weight of phosphorus pentoxide
g. at. wt. of P = gram atomic weight of phosphorus
Three replicate determinations were obtained for each different fertilizer. The comparison between the value percentage P2O5 and to the commercial fertilizer were done.
Determination of Nitrogen in the fertilizer
The nitrogen in the fertilizer samples was determined by the Kjeldahl method. A mass of 1.0 g of the diammonium phosphate fertilizer prepared was weighed and transferred to a two-necked digestion flask and set up for distillation. A receiver flask containing25.0 ml of 4% boric acid solution was attached to the distillation unit. About 50.0 cm3 of NaOH (40%) solution added to the fertilizer in the flask to initiate evolution of ammonia which was absorbed into the boric acid in the receiver flask. Distillation took 20 minutes after which the receiver flask was removed; 5 drops of screened methyl red indicator solution were added to the distillate receiver flask and titrated against 0.05 M sulphuric acid to a grey end point. The procedure was repeated three times and results averaged. Two blank titrations were run and the average blank value used for subsequent calculations. The content of nitrogen, (WN), in milligrams per gram, was calculated using equation (2) shown below:
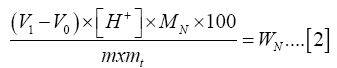
Where,
V1= The volume, in ml of the sulphuric acid used in the titration of the sample
V0 = The volume, in ml of the sulphuric acid used in the titration of the blank test
[H+] = The concentration of H+ in the sulphuric acid in moles per litre (e.g. if 0.01 mol/l sulphuric acid is used, [H+] = 0.01 mol/l)
MN = The molar mass of nitrogen, in grams per mole (=14)
m = The mass of test sample
mt = The dry residue, expressed as g/100 g on the basis of oven dried material according to the standard of the special material.
Determination of Moisture in the fertilizer
The moisture content of the fertilizer prepared was determined gravimetrically [18]. About 50 g of the sample was transferred to a weighing dish and first dried in a desiccator for twenty four hours to a constant mass before subjecting it to the oven temperature. It is believed that the sample would “crawl” if dried rapidly hence cause inaccurate results [18]. The sample was then placed in an oven set at 110°C for five hours and dried to a constant mass. It was then transferred to a desiccator for cooling. Five replications were done using the same procedure. The% moisture content was calculated using the equation (3) as shown:
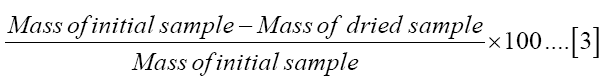
Efficacy evaluation of diammonium phosphate.
This was evaluated by applying the fertilizer to tomato plants in a greenhouse and monitoring growth parameters including, height, length of leaf, width of leaf and length of rootover a period of twelve weeks. Commercial fertilizer was used as positive control. Tomatoes grown without fertilizer were used as the negative control.
This section presents results for extraction of bone phosphate into phosphoric acid and the reaction of bone phosphate enriched phosphoric acid with ammonia to give diammonium phosphate fertilizer, characterization of the fertilizer prepared and determination of the efficacy of the same in the growing of tomatoes.
Extraction of bone phosphate
The evaluation of the effect of change of concentration of phosphoric acid on bone dissolution was as shown in Table 1. Dissolution of bones was not complete when concentrations of 0.1, 0.2, 0.25 M H3PO4 was used. Complete dissolution was observed when 0.275 M and 0.3 M were used. This indicated that the extent of dissolution was directly proportional to the concentration of phosphoric acid used. The mass of dissolved bones in the solvent was expressed as the difference between the initial mass of bones weighed before the experiment and the mass of unreacted bones. The calculation done for the dissolved bones gave the following results% P (19.45)% P2O5 (44.58) and concentration of phosphoric acid was raised from 0.275 M to 4.58 M signifying significant phosphate enrichment.
| Molarity of phosphoric acid (g/l) | Bones: Volume of acid ratio (g: ml) | Acid concentration after dissolution (g/l) | Mass of unreacted bones (g) | % P in the filtrate | Concentration of H2SO4 used (g/l) | |||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 10:35 | 0.135 | 4.3 | 19.41 | 6 | |||
| 0.2 | 10:35 | 0.241 | 4 | 19.39 | 6 | |||
| 0.25 | 10:35 | 0.29 | 4 | 19.78 | 6 | |||
| 0.27 | 10:35 | 0.355 | 0 | 19.84 | 6 | |||
| 0.3 | 10:35 | 0.356 | 0 | 19.83 | 6 | |||
Table 1. Change of acid concentration on bone dissolution.
Synthesis and characterization of diammonium phosphate fertilizer
The yield of the dissolved phosphorus in g/l was determined as a mass percentage of the phosphorus content of the crushed bone which was equivalent to 28.11% P2O5. Reaction of phosphoric acid with ammonia in the ratio of 1.4:1 was a very exothermic reaction with temperatures recorded reaching 95 oC. The reaction was exothermic with the highest temperature recorded during the reaction as 95°C but the crystals formed at 51°C. After the reaction, the temperatures of the reaction vessel were allowed to cool to 26°C. The crystals were filtered from the mother liquor and dried in air giving a mass of 3170.60 g of diammonium phosphate, equivalent to 48.06% yield. The calibration curve for the standard solutions was calibrated and the equation used to calculate the concentrations of PO43- in the fertilizer prepared and commercially obtained fertilizer. The values for PO43- obtained were converted to Total Phosphorus by multiplying with 0.3261 whereas; Total Phosphorus was converted to P2O5 by multiplying the values obtained by 2.2915. Table 2 shows the average absorbance and concentrations of the standard solutions while Figure 1 shows the calibration curve.
| Volume (ml) of standards | Average absorbance | Concentration of PO43- (ppm) |
|---|---|---|
| 1 | 0.2295 | 0.558 |
| 2 | 0.3904 | 1.0204 |
| 3 | 0.5605 | 1.5092 |
| 4 | 0.7313 | 2 |
| 5 | 0.9185 | 2.5379 |
| 6 | 1.099 | 3.0566 |
| 7 | 1.255 | 3.5049 |
| 8 | 1.397 | 3.9129 |
Table 2. Average absorbance and concentrations of standard solution.
Concentration of phosphate levels in the fertilizer was determined using the equation of the calibration curve and results recorded in Table 3.
| Synthesized DAP | Absorbance | Concentration (ppm) | % P2O5 | Commercial DAP | Absorbance | Concentration (ppm) | % P2O5 |
|---|---|---|---|---|---|---|---|
| Sample 1 | 0.2428 | 0.5964 | 44.56 | Sample 1 | 0.2467 | 0.6082 | 45.45 |
| Sample 2 | 0.2433 | 0.5976 | 44.66 | Sample 2 | 0.2471 | 0.6086 | 45.48 |
| Sample 3 | 0.2426 | 0.5957 | 44.52 | Sample 3 | 0.247 | 0.6083 | 45.46 |
| Average | - | - | 44.58 | Average | - | - | 45.46 |
| Rsd (%) | - | - | 0.23 | Rsd (%) | - | - | 0.23 |
Table 3. Levels of % P2O5 in Synthesized DAP and commercial DAP.
Synthesized DAP contained 44.58% P2O5 as compared to 45.46% P2O5 for commercial DAP. The difference could be accounted for by the fact that bones contain 15–19% P and rock phosphate contains 15 ± 1% P [12]. The% N levels in the prepared DAP and commercial DAP were analysed using Kjeldahl process and results recorded in Table 4.
| Laboratory fertilizer | Msample (g) | Vsample (ml) | %N | Commercial fertilizer | Msample (g) | Vsample (ml) | %N |
|---|---|---|---|---|---|---|---|
| Sample 1 | 0.6034 | 14.73 | 17.07 | Sample 1 | 0.668 | 16.55 | 17.35 |
| Sample 2 | 0.5787 | 14.22 | 17.21 | Sample 2 | 0.6979 | 17.14 | 17.2 |
| Sample 3 | 0.6045 | 14.94 | 17.31 | Sample 3 | 0.6758 | 16.78 | 17.39 |
| Average | - | - | 17.19 | Average | - | - | 17.31 |
| Rsd (%) | - | - | 0.77 | Rsd (%) | - | - | 0.77 |
Table 4. Levels of % nitrogen in synthesized and commercial DAP.
Synthesized DAP contained 17.19% N as compared to commercial DAP (17.31% N). This could probably be as a result of the effect of poor storage facilities for the synthesized DAP where some levels of ammonia were lost. The values for the moisture content for the fertilizers determined were recorded in Table 5.
| Variables | Mean Weight of Wet Fertilizer (g) | Mean Weight of Dry Fertilizer (g) | % Moisture content |
|---|---|---|---|
| Synthesized DAP | 50 | 23.5 | 0.53 ± 0.14 |
| Commercial DAP | 50 | 30.5 | 0.39 ± 0.06 |
Table 5. % Moisture content in synthesized and commercial DAP.
The synthesized fertilizer contained a higher percentage of moisture (0.53 ± 0.14) as compared to the commercial fertilizer (0.39 ± 0.06). This could be attributed to the solubility levels of the fertilizer. It is assumed that commercial fertilizer contained low moisture content due to the treatment to which it was subjected to in the process of manufacture.
Efficacy of the prepared DAP in tomato growth
This was evaluated by applying the fertilizer to tomato plants in a greenhouse and monitoring growth parameters including, height, length of leaf, width of leaf and length of rootover a period of twelve weeks. Commercial fertilizer was used as positive control. Tomatoes grown without fertilizer were used as the negative control.
Height of tomato plants
The changes in height of tomatoes planted with lab fertilizer, commercial fertilizer and without fertilizer were measured over a period of twelve weeks. The plant heights were measured weekly for the first two weeks and then every two weeks until the twelfth week. Table 6 summarises the results obtained.
| Fertilizer/Plant | Root Length (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W4 | W6 | W8 | W10 | W12 | Margin | |
| Average change in root length (Synthesized fertilizer) | 2.44 ± 0.423c | 13.12 ± 0.423c | 14.6 ± 0.423c | 16.12 ± 0.423c | 17.16 ± 0.423c | 18.0 ± 0.423c | 19.0 ± 0.423c | 13.917 ± 0.027 |
| Average change in root length (commercial fertilizer) | 2.5 ± 0.423c | 13.12 ± 0.423c | 14.70 ± 0.423c | 15.94 ± 0.423c | 17.20 ± 0.423c | 18.96 ± 0.423c | 19.62 ± 0.423c | 14.388 ± 0.027 |
| Average change in root length (Zero fertilizer) | 2.02 ± 0.423c | 2.74 ± 0.423c | 3.3 ± 0.423c | 3.98 ± 0.423c | 4.64 ± 0.423c | 5.16 ± 0.423c | 5.58 ± 0.423c | 14.588 ± 0.027 |
Table 6. Change in heights of tomato plants with time.
It can be seen from the table that the height of the tomato plants which were planted with synthesized fertilizer increased with time from week one reaching max at week twelve. The height attained was not significantly different from what was observed with tomatoes planted with commercial fertilizer (p-values = 0.000 < 0.05). The height attained in tomatoes planted with synthesized fertilizer was significantly different from the height attained in tomatoes planted without fertilizer (i.e. the negative control). There was increase in heights of tomato plants grown without fertilizers up to week four then reduction in growth as shown by the rates between week six and twelve. This could be attributed to decreasing availability of native phosphorus in the soil. Plants grown with commercial fertilizer gave the largest increase in height, although the increase was not significantly different from change in heights of plants grown with laboratory prepared fertilizer. The relatively high amounts of% N and% P2O5 in commercial fertilizer (17.31, 45.46) respectively could be the main reason for the consistently better performance of commercial DAP. The highest height was recorded at week twelve. There was significant decrease in the rate of increase in plant heights after week six in all treatments. This could probably be due to decrease in the levels of nitrogen and phosphorus as plants grew. The rate of increase in height was calculated as an average of the values between two weeks divided by time. For the first interval (between week one and two) growth rate was calculated as the increase in total length per day.
Root length of the tomatoes
The changes in root length of tomatoes planted with synthesized fertilizer, commercial fertilizer and with no fertilizer were measured over a period of twelve weeks. The root length was measured weekly for the first two weeks and then every two weeks until the twelfth week. Table 7 summarizes the obtained results.
| Fertilizer/Plant | Tomato plant Heights (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | Margin | |
| Synthesized fertilizer | 9.59 ± 0.29c | 21.52 ± 0.29c | 31.42 ± 0.29c | 33.72 ± 0.29c | 42.11 ± 0.29c | 47.93 ± 0.29c | 51.61 ± 0.29c | 34.02 ± 0.19 |
| Commercial fertilizer | 10.27 ± 0.29c | 22.33 ± 0.29c | 28.99 ± 0.29c | 33.79 ± 0.29c | 42.446 ± 0.29c | 49.43 ± 0.29c | 53.3 ± 0.29c | 34.37 ± 0.19 |
| No fertilizer | 5.69 ± 0.29c | 10.22 ± 0.29c | 17.70 ± | 18.80 ± | 20.04 ± 0.29c | 20.04 ± 0.29c | 20.41 ± 0.29c | 16.13 ± 0.19 |
| 0.29c | 0.29c | |||||||
Table 7. Root length of tomato plants.
It can be seen from the table that the root length of the tomato plants which were planted with synthesized fertilizer increased from week one and reached maximum at week twelve. There was also increase in root lengths in plants grown with commercial fertilizer from week one to week twelve. The rate of increase in root length was more at the beginning of the planting period, that is, week one and week two than towards the end (week 10 and week twelve). This shows that roots have the greatest demand for phosphorus at early stages of root development. There was suppressed change in root length for plant grown without fertilizers with time as shown by decrease in rate of growth, an indication of phosphorus deficiency. The change in root length for plantsgrown with synthesized fertilizer was not significantly (p-values = 0.000 < 0.05) different from the change in root length in plants grown with commercial fertilizer. Amponsha [17] describes phosphorus as a stimulant for early root formation and growth that helps in absorption of essential minerals and water.
Variation of leaf length of the tomato plants
The change in leaf length of tomatoes planted with synthesized fertilizer, commercial fertilizer and with no fertilizer was measured over a period of twelve weeks. The leaf length was measured weekly for the first two weeks after transplanting and then after every two weeks until the twelfth week, Table 8 summarizes the results obtained. The leaf length continued to increase from week one to week twelve for plants grown with synthesized fertilizer, but the rate of increase of leaf length decreased with time. For example, the leaf length increased from 2.51 cm to 5.67 cm from week one to week two which was equivalent to the rate of change of 0.45 cm/day and between week two and week three, the leaf length increased from 5.67 cm to 7.54 cm which was equivalent to a rate of 0.13 cm/day. The leaf length of plants grown with commercial fertilizer also increased from week one to week twelve. The rate of increase of leaf length in cm/day also decreased with time.
| Fertilizer/Plant | Leaf Length (cm) | |||||||
|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W4 | W6 | W8 | W10 | W12 | Margin | |
| Average change in leaf length (synthesized fertilizer) | 2.51 ± 0.08 c | 5.67 ± 0.08 c | 7.54 ± 0.08 c | 9.46 ± 0.08 c | 10.55 ± 0.08 c | 11.67 ± 0.08 c | 12.42 ± 0.08 c | 8.549 ± 0.529 |
| Average change in leaf length (commercial fertilizer) | 2.39 ± 0.08 c | 5.74 ± 0.08 c | 7.94 ± 0.08 c | 9.48 ± 0.08 c | 10.83 ± 0.08 c | 11.88 ± 0.08 c | 13.06 ± 0.08 c | 8.763 ± 0.529 |
| Average change in leaf length (zero fertilizer) | 2.10 ± 0.08 c | 2.92 ± 0.08 c | 3.92 ± 0.08 c | 4.82 ± 0.08 c | 5.24 ± 0.08 c | 5.60 ± 0.08 c | 5.88 ± 0.08 c | 4.355 ± 0.529 |
Table 8. Leaf length of tomato plants.
For example, between week one and week two, the leaf length increased from 2.10 cm to 2.92 cm giving an increase in rate as 0.11 m/day whereas between week two and week three, there was an increased rate of 0.071 cm/day. Plants to which the fertilizers were applied yielded higher leaf length values as compared to plants where no fertilizer was applied. For the two fertilizers (synthesized and commercial), plants grown with commercial fertilizers yielded higher leaf length values as compared to plants grown with laboratory fertilizer, though, the two fertilizers were not significantly different (p-values = 0.000 < 0.05). Previous research has shown that diammonium phosphate fertilizer has nitrogen which has a promoting effect on the leaf growth [19].
Leaf width of the tomatoes
The change in leaf width of tomatoes planted with synthesized fertilizer, commercial fertilizer and with no fertilizer was measured over a period of twelve weeks. The leaf width was measured weekly for the first two weeks and then every two weeks until the twelve weeks. Table 9 summarizes the results obtained.
| Fertilizer/Plant | Leaf Width (cm) Periods | |||||||
|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W4 | W6 | W8 | W10 | W12 | Margin | |
| Average change in leaf width (synthesized fertilizer) | 1.24 ± 0.043 c | 2.8 ± 0.043 c | 3.74 ± 0.043c | 4.60 ± 0.043c | 5.24 ± 0.043 c | 5.74 ± 0.043 c | 6.20 ± 0.043 c | 4.222 ± 0.028 |
| Average change in leaf width (commercial fertilizer) | 1.2 ± 0.043c | 2.84 ± 0.043c | 3.94 ± 0.043c | 4.7 ± 0.043c | 5.36 ± 0.043c | 5.92 ± 0.043c | 6.5 ± 0.043c | 4.348 ± 0.028 |
| Average change in leaf width (zero fertilizer) | 1.02 ± 0.043c | 1.44 ± 0.043c | 1.96 ± 0.043c | 2.3 ± 0.043c | 2.6 ± 0.043c | 2.78 ± 0.043c | 2.92 ± 0.043c | 2.145 ± 0.028 |
Table 9. Variation of leaf width of tomato plants.
The leaf width progressively increased from week one to week twelve for plants grown with synthesized fertilizer. The rate of increase in leaf width decreased with time. For example, between week one and week two, the rate was 0.82 cm/day, between week two and week three, the rate was 0.078 cm/day and between week three and week four, the rate was 0.061 cm/day. The leaf width of plants grown with commercial fertilizer also increased from week one to week twelve. The rate of increase in leaf width also decreased from week 1 to week twelve, for example, between week one and week two, the rate of growth was 0.23 cm/ day, between week two and week three, the rate was 0.078 cm/day and between week three and week four, the rate was 0.054 cm/day.
The leaf width of plants grown without fertilizer also increased marginally. For example, between week one and week two, the leaf width increased from 1.02 cm to 1 .44 cm giving an increased rate of 0.06 cm/day and between week two and week three, there was an increased rate of 0.03(cm/day). Plants to which the fertilizers were applied yielded higher leaf width values as compared to plants where no fertilizer was applied. Plants grown with commercial fertilizer showed higher leaf width values than plants grown with synthesized fertilizer, but the leaf widths were not significantly different at (p-values = 0.000 < 0.05). Previous research [20] has shown that the width of tomato leaves increase over time when planted with diammonium phosphate fertilizer. Leghari [21] also observes that a significant reduction in the rate of increase in leaf width is as a result of reduced phosphorus over time which leads to reduction in cell division and leaf expansion.
The DAP fertilizer was synthesized, characterized and used to grow tomatoes in a greenhouse with commercial DAP as the positive control. Tomato plants grown without fertilizers served as the negative control. Based on the results discussed in this manuscript, the following conclusions can be made:
i) Bone phosphate was successfully extracted by acid hydrolysis thereby enriching commercial phosphoric acid from 0.275 M phosphoric acid to 4.58 M.
ii) DAP fertilizer was successfully prepared by reacting NH3 from hydrolysis of Li3N and bone phosphate enriched phosphoric acid.
iii) The DAP obtained was as efficacious as the commercial DAP in growing tomatoes in the green house.