e-ISSN: 2319-9849
e-ISSN: 2319-9849
Chemistry Department, Faculty of Science, Damietta University, New Damietta, Egypt
Received date: 19/08/2015; Accepted date: 21/10/2015; Published date: 23/10/2015
Visit for more related articles at Research & Reviews: Journal of Chemistry
Vilsmier-Haack formylation of 5-bromo-2,3-diphenyl indole afforded 6- formyl derivative which was subjected to various condensation reactions that finally gave heterocyclic rings bounded to the starting compound at position 6. Biological screening of the synthesized compounds revealed that some of it has antibacterial activity.
2,3-Diphenylindole, 5-bromo-2,3- diphenyl indole, 6-formyl derivative, Antibacterial activity, Biological screening.
The importance of indole nucleus is well documented in the field of pharmaceutical chemistry as well as plant and animal biochemistry [1-3]. Various biological activities like hallucinogenic, hypnotic, sedative and antidepressant have been found to be associated with the indole derivatives [4-6]. The ability of 5-hydroxy tryptamine and biologically active natural ergot alkaloids to interrupt pregnancy and produce hemorrhage in placenta has prompted many chemists and pharmacologists to develop new indole heterocycles as potential antifertility agents [7,8]. On the other hand, the interesting biological activities of a large number of indole thiocarbamoylhydrazine 4-thiazolidinone and thiazoline derivatives are well known [9-11]. Various analogues of pyrimidines and thiopyrimidines showed significant chemotherapeudcal activities [12,13]. Based on these findings and in continuation to our work directed towards the synthesis of heterylindoles of potential biological activities [14-16], it was of interest to synthesize new indole derivatives by incorporating these biogenic moieties with a hope of increasing the drug action.
Vilsmeier-Haack formylation of compound (I) with dimethylformamide and phosphorus oxychloride gave 2,3-diphenyl-5- bromo-6-formyl -1-H-indole (II). The IR spectrum of compound (II) showed an absorption band at 1730 cm-1 characteristic for the carbonyl function of the formyl group.
1H NMR spectrum (250 MHz, DMSO) revealed signals at 10.30 (s, 1H, CHO group), 7.22-7.80 (m, 12H, Ar-H), 10 (s, 1H, =NH indole). The formylation is directed to position 6 not to any other position since position 6 of compound (I) is the most reactive centre [17-19].
Reaction of 2,3-diphenyl-5-bromo-6-formyl-1-H-indole (II) with semicarbazide hydrochloride and thiosemicarbazide hydrochloride gave the corresponding 2-[(2-3- diphenyl-5-bromo-l H-indol-6-yl)-methylidene] hydrazine carbamide (III) and 2-[(2-3 –diphenyl-5-bromo-l-H-indol-6yl) methylidene]hydrazine thiocarbamide (IV), respectively which their IR spectra were characterized by the disappearance of strong absorption band at 1730 cm-1 of the formyl group and the appearance of absorption band at 1690 cm-1 due to -CH=N- group.
Semicarbazone (III) and thiosemicarbazone (IV) were cyclized with ferric chloride in boiling ethanol affording 5-[(2,3-diphenyl- 5-bromo -1-H -indol- 6-yl)]-1,2,4-triazolidin-3-one (V) and 5-[(2,3diphenyl-5-bromo -1 -H -indol- 6-yl)]-1,2,4- triazolidine-3-thione (VI), respectively. The presence of sharp absorption bands at 1726 cm-1 and 1681 cm-1 in the IR spectra of compounds (V) and (VI) was attributed to cyclic CONH group and CSNH group, respectively.
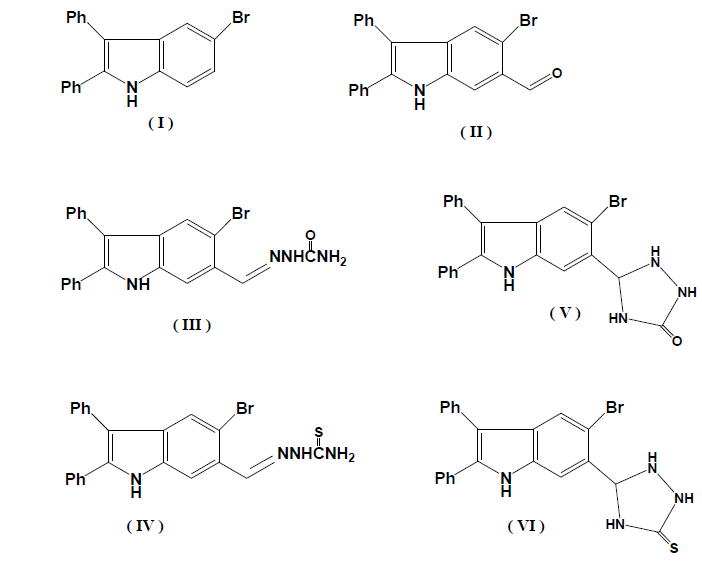
Condensation of (II) with diethyl malonate in presence of pipridine afforded diethyl 2-[(2,3-diphenyl-5-bromo-1-indol-6-yl) methylidene] malonate (VII). The IR spectrum showed an absorption band at 1721 cm-1 for C=O group of α,β-unsaturated ester. The reaction between (VII) with urea and thiourea gave 5-[(2,3-diphenyl-5-bromo-1-H-indol-6-yl)methylidene]-barbituric acid (X) and 5-[(2,3-diphenyl-5-bromo-1-H-indol-6-yl)methylidene]-thiobarbituric acid (XI), respectively (Scheme 1). The IR spectrum of (X) revealed absorption bands at 1766 cm-1, (C=O groups), 3339 cm-1,(NH indole) and 3325 cm-1, NH (imino group). The 1H NMR spectrum (250 MHz, DMSO) revealed signals at 11.08(s, 2H, NH imino group), 9.5(s, 1H),7.1-7.48 (m, 12H, Ar-H), 10.15 (s, 1H, =NH indole). The IR spectrum of (XI) revealed absorption bands at 1690, 1657,1644,3337, and 3667 cm-1 (C=O groups), C=S, ( NH indole) and (NH group) respectively. The 1H NMR spectrum revealed signals at 8.0 (s, 2H, NH imino group), 8.30 (s, 1H),7.1- 7.48 (m, 12H, Ar-H), 10.15 (s, 1H, =NH indole).
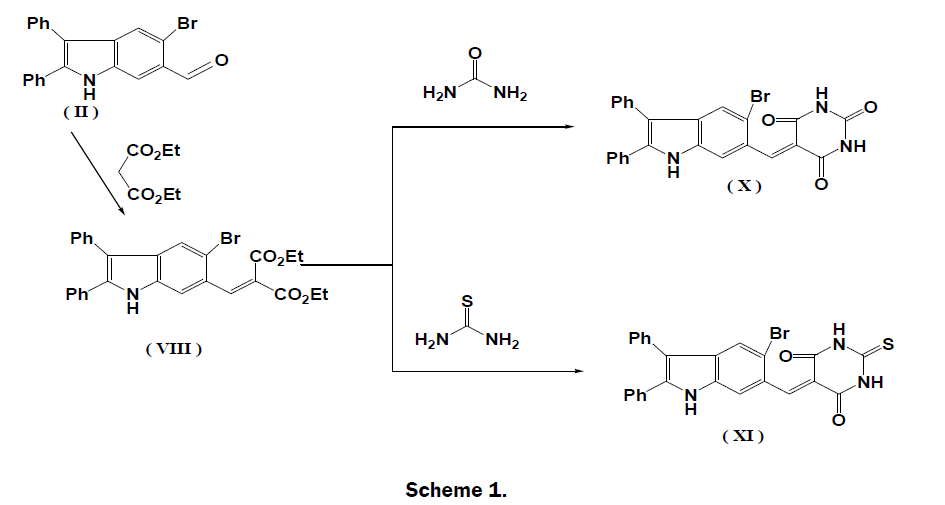
Although, it is known that the reaction of arylidines and hydrazine hydrate resulted in the formation of heterocyclic compounds but, we have out that the reaction of arylidine derivatives, (VII), (VIII) and (IX) with hydrazine hydrate gave the same product, hydrozone derivative (XII), not the expected cyclization products (XIII), (XIV) and (XV) (Scheme 2). The IR spectrum of (XII) showed an absorption bands at 1689 and 3404 cm-1 due to -CH=N- and NH2 groups.
Some of synthesized compounds were tested for their biological activity. It have been tested against two types of Bactria (Bacillus subtilis and Escherichia coli). The biological screening was tested by Miss. Faten Heryher of Microbiology Lab, Botany Department, Faculty of Science, New Damietta, Egypt (Table 1).
| Compound | BactericidalÅ | Inhibition zone(mm) | |
|---|---|---|---|
| B.S | E.C | ||
| ( I ) | (-) | (-) | (-) |
| ( II ) | (-) | (-) | (-) |
| ( III ) | (+) | (+) | (23) |
| ( IV ) | (+) | (+) | (25) |
| ( V ) | (+) | (+) | (27) |
| ( VI ) | (+) | (+) | (28) |
Table 1. The following (Table 1) shows the results of some examples of the tested compounds.
Melting points (uncorrected) were determined on Stuart Scientific Melting Point. IR, 1H NMR and elemental analysis (Table 2) were performed at Microanalysis Unit (Cairo University) and at National Research Centre, Dokki, Cario, Egypt.
| Compound | Yield (%) | M.P (°C) | Molecular Formula (M.Wt.) | Elemental Analysis |
|---|---|---|---|---|
| (II) | 83 | 125 | C21H14BrNO(376) | calculatedC=67.02%,H=3.72%,N=3.72%, found C=67.04%,H=3.75%,N=3.76%, |
| (III) | 75 | 145 | C22H17BrN4O(433) | calculatedC=60.97%,H=3.90%,N=12.91%, found C=60.98%,H=3.95%,N=12.93%, |
| (IV) | 73 | 146 | C22H17BrN4S(449) | calculatedC=58.79%,H=3.78%,N=12.47% found C=58.80%,H=3.81%,N=12.46% |
| (V) | 70 | 160 | C22H17BrN4O(433) | calculatedC=60.96%,H=3.92%,N=12.93% found C=60.98%,H=3.96%,N=12.97% |
| (VI) | 73 | 178 | C22H17BrN4S(449) | calculatedC=58.79%,H=3.78%,N=12.47% found C=58.80%,H=3.81%,,N=12.46% |
| (VII) | 68 | 143-47 | C28H24BrNO4(518) | calculatedC=64.86%,H=4.63%,,N=2.70% found C=64.87%,H=4.67%,N=2.71% |
| (VIII) | 65 | 132-138 | C26H19BrN2O2(471) | calculatedC=66.24%,H=4.03%,N=5.94% found C=66.25%,H=4.06%,N=5.96% |
| (IX) | 68 | 145-48 | C24H14BrN3(424) | calculatedC=67.92%,H=3.30%,N=9.90% found C=67.94%,H=3.33%,N=9.91% |
| (X) | 75 | 120 | C25H16BrNO4(486) | calculatedC=61.72%,H=3.29%,N=8.64%, found C=61.74%,H=3.32%,N= 8.66%, |
| (XI) | 74 | 100 | C25H16BrN3O2S(502) | CalculatedC=59.76%,H=3.18%,N=8.3 found C=59.77%,H=3.21%,N= 8.40% |
| (XII) | 65 | 71 | C21H16BrN3(390) | calculated C=64.61%,H=4.10% ,N=10.76% found C=64.63%,H=4.20% ,N=10.77% |
Table 2: The following table shows the results of some examples of the calculated compounds.
2,3-diphenyl -5-bromo –1- H- 6-formyl indole (II)To a cold solution of (I) (7,025 gm, 20 mmole) in dimethyl formamide (4.2 ml, 0.0542 mole ), phosphorus oxychloride (3.5 ml, 0.038 mole ) was added. The reaction mixture was heated on boiling water bath for 2 hrs left to cool to room temperature, the reaction mixture was poured into ice-cold water and neutralized with sodium acetate trihydrate (100 gm /175 ml H2O) solution the solid so obtained was crystalized from ethanol as yellowish crystals.
2-[(2,3-diphenyl-5-bromo-l-H-indol-6-yl)-methylidene]hydrazine carbamide (III)To a suspension of (II) (1.1 gm, 2.9 mmole ) in 20 ml ethanol 95%, (0.42 gm, 2.9 mmole ) of semicarbazide hydrochloride and sodium acetate trihydrate (0.48 gm, 3.5 mmole ) was added, the reaction mixture was refluxed for 4 hrs., the separated semicarbazone was filtered off and crystallized from ethanol as a white crystals.
2-[(2,3-diphenyl-5-bromo-l-H-indol-6-yl)-methylidene]hydrazine thiocarbamide (IV)To a suspension of (II) (1.1 gm, 2.9 mmole ) in 20 ml ethanol 95%, (0.46 gm., 2.9 mmole ) of thiosemicarbazide hydrochloride and sodium acetate trihydrate (0.48 gm, 3.5 mmole ) was added, the reaction mixture was refluxed for 4 hrs The separated thiosemicarbazone was filtered off and recrystallized from ethanol.
5-[(2,3-diphenyl-5-bromo-1-H-indol-6-yl)]-1,2,4-triazolidin-3-one (V)To a solution of semicarbazone (III) (1 mmole ) in 30 ml ethanol, a solution of (2 mmole ) of ferric chloride in 20 ml ethanol was added and the reaction mixture was refluxed for 2 hrs. The reaction mixture was left to cool to room temperature, and the separated solid was filtered off, washed with water, dried and crystallized from ethanol.
5-[(2,3-diphenyl-5-bromo-1-H -indol-6-yl)]-1,2,4-triazolidin-3-thione (VI)To a solution of thiosemicarbazone (IV) (1 mmole ) in 30 ml ethanol, a solution of (2 mmole ) of ferric chloride in 20 ml ethanol was added and the reaction mixture was refluxed for 2 hrs, the reaction mixture was left to cool to room temperature, the separated solid was filtered off, washed with water and recrystalized from ethanol.
Diethyl-2-[(2,3-diphenyl–5-bromo–1-H- indol-6-yl)methylidene] malonate (VII)suspension of the aldehyde compound (II) (2 gm, 5 mmole) in ethanol 30 ml (95%), 1 ml piperidine and the equimolecular weight of diethylmalonate was refluxed for 4 hrs., the reaction mixture was cooled to room temperature, the separated solid was filtered off and recrystallized from the ethanol, as yellow crystals.
Ethyl-α-cyano-β-[(2,3-diphenyl-5-bromo–1-H-indol-6-yl)] acrylate (VIII)A suspension of the aldehyde compound (II) (2 gm, 5 mmole) in ethanol 30 ml (95%), 1 ml piperidine and the equimolecular weight of ethylcyanoacetate was refluxed for 4 hrs., the reaction mixture was cooled to room temperature, the separated solid was filtered off and recrystallized from the ethanol.
α-cyano-β-[(2,3-diphenyl-5-bromo–1-H-indol-6-yl) methylidene] acrylonitrile (IX)A suspension of the aldehyde compound (II) (2 gm, 5 mmole) in ethanol 30 ml (95%), 1 ml piperidine and the equimolecular weight of malononitrile was refluxed for 4 hrs., the reaction mixture was cooled to room temperature, the separated solid filtered off and recrystalized from ethanol.
5-[(2,3-diphenyl–5-bromo–1-H-indol-6-yl)methylidene] barbituric acid (X)To a solution of sodium ethoxide (0.3 gm, 0.013 mol of clean sodium soluble in 6 ml absolute ethanol ), (IX) (0.5 gm,0.9 mmole ) and alcoholic urea solution (0. 67 gm,10 mmole dry urea, 0.05 ml of hot absolute ethanol) was added . The reaction mixture was stirred under reflux for 30 min at room temperature then for 7 hrs. In oil bath at 110°C, poured into 100 ml water at (50°C), treated with Conc. hydrochloric acid with stirring until the solution become acidic (3 ml Conc. HCl ) and was left in refrigerator overnight . The separated solid was filtered off, washed with cold water, dried and recrystalized from ethanol.
5-(2,3-diphenyl–5-bromo–1-H-indol-6-yl) methylidene] thiobarbituric acid (XI)To a solution of sodium ethoxide (0.3 gm, 0.013 mol of clean sodium soluble in 6 ml absolute ethanol ), (IX) (0.5 gm, 0.9 mmole ) and alcoholic thiourea solution (0. 67 gm, 10 mmole dry thiourea, 0.05 ml of hot absolute ethanol ) was added. The reaction mixture was stirred under reflux for 30 min at room temperature then for 7 hrs in oil bath to 110°C. Then was poured into 100 ml water at (50°C), the reaction mixture was treated with Conc. Hydrochloric acid with stirring until the solution is acidic (3 ml Conc. HCl), left it in refrigerator overnight. The separated solid was filtered off and washed with cold water, the solid obtained was crystallized from ethanol as red crystals.
2,3-diphenyl-5- bromo–1-H-indol-6-formaldehyde hydrazone (XII)A suspension of (VII), (VIII) and (IX) (1 gm, 2 mmole) in 20 ml ethanol 95% and hydrazine hydrate (1.5 ml) was refluxed for 4 hrs. the separated solid was filtered off, one product (XII) was obtained .