e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, Saurashtra University, Rajkot, Gujarat, India
Received Date: 07/12/2017; Accepted Date: 19/12/2017; Published Date: 09/01/2018
Visit for more related articles at Research & Reviews: Journal of Chemistry
Naphtho[2,1-b]furan-2-carbohydrazide (2) prepared from reaction between ethyl naphtho[2,1-b]furan-2-carboxylate (1) and hydrazine hydrate, then compound (2) on reaction with CS2/KOH gives 5- (naphtho[2,1-b] furan-2-yl)-1,3,4-oxadiazole-2(3H)-thione (3), Which on mannich reaction followed by reaction with benzoic hydrazine gives different N-(1-((dialkylamino)methyl)-3-(naphtho[2,1-b]furan-2-yl)-5- thioxo-1H-1,2,4-triazol-4(5H)-yl)benzamide (4a-e). With the help of analytical and spectral data, the structure of these compounds were detected. Antibacterial and antifungal activities of the synthesized compounds were studied.
Triazole, Methylation, N-butylation, Suzuki-Miyaura coupling, Solvent, Spectroscopy
Literature survey reveals that various 1,2,4-triazole derivatives display significant biological activities such as bactericidal, [1] diuretic, [2] fungicidal, [3] herbicidal, [4] insecticidal and acaricidal, [5] plant growth regulator, [6] anticancer, [7] 5-lipoxygenase inhibitors [8] and anti-HIV, [9] antileshmanial, [10] antitumor [11] activities. Platinum(II) complexes comprising 1,2,4-triazoles as ligands show antitumor activity similar to cis-platin. [12] Furthermore, ruthenium(III) complexes of 1,2,4-triazoles are promising as potential drugs in anticancer treatment as alternative to the approved platinum-based anticancer drugs. [13] 1,2,4-Triazoles such as rizatriptan as agents for acute treatment of migraine headaches are commercially available drugs; [14] however, they are also still a topic of intensive research [15]. Keeping in mind the pharmacological applications of this class of compounds and with a view to further assess the pharmacological profile of this class of compounds, the present section incorporates synthesis of thirty novel analogues of 1,2,4-triazole derivatives.
Materials and Methods
Melting points were determined in open capillary tubes and are uncorrected. Formation of the compounds was routinely checked by TLC on silica gel-G plates of 0.5 mm thickness and spotsvisualization was made with UV light (254 and 365 nm) or with an iodine vapor. IR spectra were recorded on Shimadzu FT-IR-8400 instrument using KBr pellet method. Mass spectra were recorded on Shimadzu GCMS-QP-2010 model using Direct Injection Probe technique. 1H NMR and 13C NMR were determined in DMSO-d6 solution on a Bruker Ac 400 MHz spectrometer. Elemental analyses of the all the synthesized compounds were carried out on Elemental Vario EL III Carlo Erba 1108 model and the results are in agreement with the structures assigned.
General Procedure
In first step, 4-bromobenzothioamide (Int-1) was prepared from 4-bromobenzonitrile by stirring with sodium hydrogensulphide and magnesium chloride in DMF, which followed by methylation afforded the S-methyl benzothioamide derivative (Int-2). The condensation of S-methyl benzothioamide derivative (Int-2) and 2,2,2-trifluoroacetohydrazide at 150°C in DMF afforded 3-(4-bromophenyl)-5-(trifluoromethyl)-1H-1,2,4-triazole (Int-3) in good yield, which was subjected to N-butylation at 100°C in DMF presence of K2CO3 base to afford 3-(4-bromophenyl)-1-butyl-5-(trifluoromethyl)-1H-1,2,4-triazole (Int-4). In the final step, (Int-4) was subjected to Suzuki-Miyaura reaction with various aryl boronic acids in the presence of palladium catalyst, TBAB, K2CO3 and DMF:water as a solvent at 120°C to afford the final products 3-([1,1'-biphenyl]4-yl)-1-butyl-5-(trifluoro methyl)-1H-1,2,4-triazole (P116-P125) in moderate to high yield. The structures of all newly substituted-triazole derivatives were identified by Mass, IR, 1H NMR, 13C spectroscopy.
1-butyl-3-(4'-ethyl[1,1'-biphenyl]-4-yl)-5-(trifluoromethyl)-1H-1,2,4-triazole (P 117)
Yield=85%, m.p. 137-139 ˚C; IR (KBr) cm-1: 3088, 2960, 2877, 1438, 1340, 1166, 1123, 893 1H NMR (400 MHz, CDCl3) δ ppm: 0.66-1.01(t, 6H), 1.25-1.44 (m, 4H), 1.88-1.95 (m, 2H), 4.14-4.22 (t, 2H), 7.54-7.56 (d, 4H, Ar-H), 7.95-7.97 (d, 2H, Ar–H); 13C NMR (CDCl3) δ:14.55, 15.71, 19.78, 28.95, 33.59, 50.12, 122.13, 125.33, 128.12, 132.12, 138.46, 142.14, 142.82, 143.20, 143.44, 161.37; MS (m/z): 373, Anal. Calcd. for C21H22F3N3: C, 67.55; H, 5.94; F, 15.26; N, 11.25%; Found: C, 67.05; H, 5.71; F, 14.97; N, 11.02%.
1-butyl-3-(4'-ethoxy[1,1'-biphenyl]-4-yl)-5-(trifluoromethyl)-1H-1,2,4-triazole (P118)
Yield=79%, m.p. 139-141˚C; IR (KBr) cm-1: 3036, 2962, 2875, 1599, 1510, 1431, 1338, 1259, 1166, 1128, 839, 510; 1H NMR (400 MHz, CDCl3) δ ppm: 0.95-0.99 (t, 3H), 1.26-1.29 (t, 3H), 1.33-1.42 (m, 2H), 1.89-1.91 (m, 2H), 2.67-2.73 (q, 2H), 4.18- 4.22 (t, 2H), 7.29-7.31 (d, 2H, Ar-H), 7.60-7.62 (d, 2H), 7.72-7.74 (d, 2H), 7.89-7.91 (d, 2H, Ar–H); 13C NMR (CDCl3) δ:13.8, 14.8, 19.9, 30.8, 44.6, 64.7, 113.2, 114.9, 128.0, 128.4, 128.5, 129.6, 136.5, 156.4, 160.0, 164.0; MS (m/z): 375, Anal. Calcd. for C21H22F3N3O: C, 64.77; H, 5.69; F, 14.64; N, 10.79; O, 4.11%; Found: C, 64.11; H, 5.60; F, 14.22; N, 10.42; O, 4.03%
3-(4'-bromo[1,1'-biphenyl]-4-yl)-1-butyl-5-(trifluoromethyl)-1H-1,2,4-triazole (P122)
Yield=91%, m.p. 135-137˚C; IR (KBr) cm-1: 2960, 2870, 1602,1600, 1462, 1307, 1168, 1217, 889, 589 1H NMR (400 MHz, CDCl3) δ ppm: 0.96-1.00(t, 3H), 1.20-1.36 (m, 2H), 1.94-2.05 (m, 2H), 4.20-4.23 (t, 2H), 7.2-7.24 (d, 2H, Ar-H), 7.62-7.64 (d, 2H, Ar–H), 7.86-7.88 (d, 2H, Ar-H), 8.00-8.20 (d, 2H, Ar-H); 13C NMR (CDCl3) δ: 13.8, 19.9, 30.9, 44.7, 122.0, 126.02, 128.40, 129.01, 132.25, 137.50, 142.08, 142.97, 143.20, 162.05, 163.0 MS (m/z): 424, Anal. Calcd. for C19H17BrF3N3: C, 53.79; H, 4.04; Br, 18.83; F, 13.43; N, 9.90%; Found: C, 52.78; H, 3.97; Br, 18.62; F, 13.05; N, 9.53%
All the synthesized compounds (P116-P125) were tested for their antibacterial and antifungal activity (MIC) in vitro by broth dilution method [16-18] with two Gram-positive bacteria Staphylococcus aureus MTCC-96, Streptococcus pyogenes-MTCC-443, two Gram-negative bacteria Escherichia coli MTCC-442, Pseudomonas aeruginosa-MTCC-441 and three fungal strains Candida albicans MTCC-227, Aspergillus Niger MTCC-282, Aspergillus clavatus MTCC-1323 taking ampicillin, chloramphenicol, ciprofloxacin, norfloxacin, nystatin, and greseofulvin as standard drugs. The standard strains were procured from the Microbial Type Culture Collection (MTCC) and Gene Bank, Institute of Microbial Technology, Chandigarh, India. The results obtained from antimicrobial susceptibility testing are depicted in Tables 1 and 2.
| Code | R | R1 | M.F. | M.W. | M.P. | Yield | Rf |
|---|---|---|---|---|---|---|---|
| P116 | 4-Br | 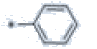 |
C19H18F3N3 | 345 | 131-133 | 89 | 0.58 |
| P117 | 4-Br |  |
C21H22F3N3 | 373 | 137-139 | 85 | 0.63 |
| P118 | 4-Br |  |
C20H20F3N3O | 375 | 139-141 | 79 | 0.61 |
| P119 | 4-Br |  |
C22H24F3N3 | 389 | 157-159 | 77 | 0.64 |
| P120 | 4-Br | 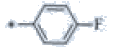 |
C19H17F4N3 | 363 | 129-131 | 83 | 0.55 |
| P121 | 4-Br | 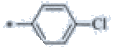 |
C19H17ClF3N3 | 380 | 151-152 | 78 | 0.57 |
| P122 | 4-Br | 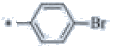 |
C19H17BrF3N3 | 424 | 135-137 | 91 | 0.58 |
| P123 | 4-Br | 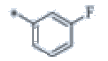 |
C19H17F4N3 | 363 | 131-133 | 81 | 0.59 |
| P124 | 4-Br |  |
C17H16F3N3S | 351 | 145-147 | 86 | 0.66 |
| P125 | 4-Br | 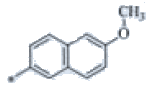 |
C24H22F3N3O | 346 | 176-178 | 77 | 0.59 |
Table 1: Physical parameters TLC Solvent system Rf: Ethyl acetate:HexaneÃÆâÃâââ¬Ãâââ¬Å4:6.
| Compounds | Minimuminhibitionconcentration(ÃÆââ¬Å¡ÃâõgmL-1) | ||||||
|---|---|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungalspecies | |||||
| S.a. | S.p. | E.c. | P.a. | C.a. | A.n. | A.c. | |
| P116 | 1000 | 250 | 500 | 250 | 1000 | 250 | 100 |
| P117 | 62.5 | 100 | 250 | 100 | 500 | 250 | 100 |
| P118 | 500 | 1000 | 1000 | 100 | >1000 | >1000 | 250 |
| P119 | 1000 | 250 | 500 | 250 | 1000 | 250 | 100 |
| P120 | 250 | 500 | 500 | 250 | >1000 | 500 | 250 |
| P121 | 1000 | 250 | 500 | 250 | 1000 | 250 | 100 |
| P122 | 250 | 500 | 500 | 250 | >1000 | 500 | 250 |
| P123 | 62.5 | 100 | 250 | 100 | 500 | 250 | 100 |
| P124 | 500 | 1000 | 1000 | 100 | >1000 | >1000 | 250 |
| P125 | 62.5 | 100 | 100 | 200 | 500 | 250 | 100 |
| Ampicillin | 250 | 100 | 100 | 100 | - | - | - |
| Chloramphenicol | 50 | 50 | 50 | 50 | - | - | - |
| Ciprofloxacin | 50 | 50 | 25 | 25 | - | - | - |
| Norfloxacin | 10 | 10 | 10 | 10 | - | - | - |
| Nystatin | - | - | - | - | 100 | 100 | 100 |
| Griseofulvin | - | - | - | - | 500 | 100 | 100 |
Table 2: Antibacterial and antifungal activity of synthesized compounds.
The minimal inhibitory concentration (MIC) values for all the newly synthesized compounds, defined as the lowest concentration of the compound preventing the visible growth, were determined by using microdilution broth method according to NCCLS standards [16].
In the present article, we report the synthesis, spectral studies, antibacterial and antifungal activities of a novel series of 1,2,4 triazole scaffold. The preliminary in vitro biological activities revealed that compounds P117, P123 and P124 exhibited moderate antibacterial activities.
We are thankful to UGC to provide Rajiv Gandhi National Fellowship for research. The authors are also gratified to Department of chemistry, Saurashtra University-Rajkot to provide research facilities.