e-ISSN: 2319-9849
e-ISSN: 2319-9849
1 Department of Chemistry, Silvassa Institute of Higher Learning, Government Aided College of Arts, Commerce and Science, DNHUSS, Silvassa – Naroli – 396235, (U. T. of D & NH) India.
2Department of Chemistry, Veer Narmad South Gujarat University, Udhna-Magdalla Road, Surat - 395007 (Gujarat) India.
3Department of Chemistry, Narmada College of Science and Commerce, Zadeshwer, Bharuch - 392011 (Gujarat) India.
4Department of Chemistry, CU Shah Science College, Ashram Road, Ahmedabad – 380014 (Gujarat) India.
Received: 12/03/2013; Revised: 29/03/2013; Accepted: 30/03/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Two novel series of mesoionic compounds containing benzothiazole and thiazole based sydnone derivatives were synthesized. All the synthesized compounds have been characterized by physical methods (melting point, TLC and elementary analysis) and by spectral techniques (IR, 1H NMR and 13C NMR).
Synthesis, Benzothiazole, Thiazole, Sydnone.
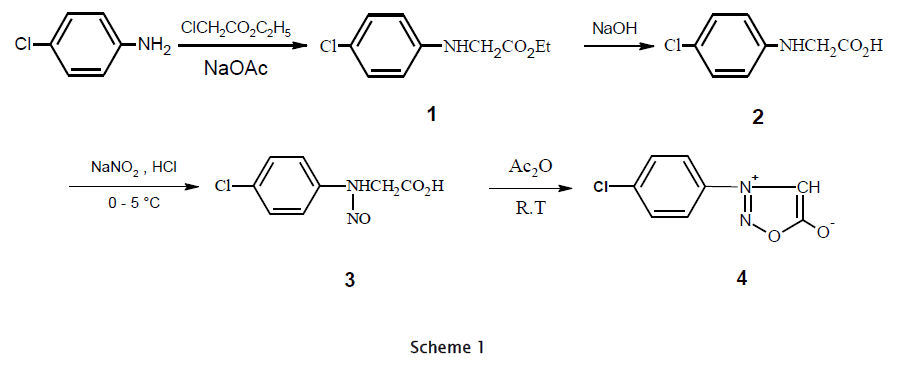
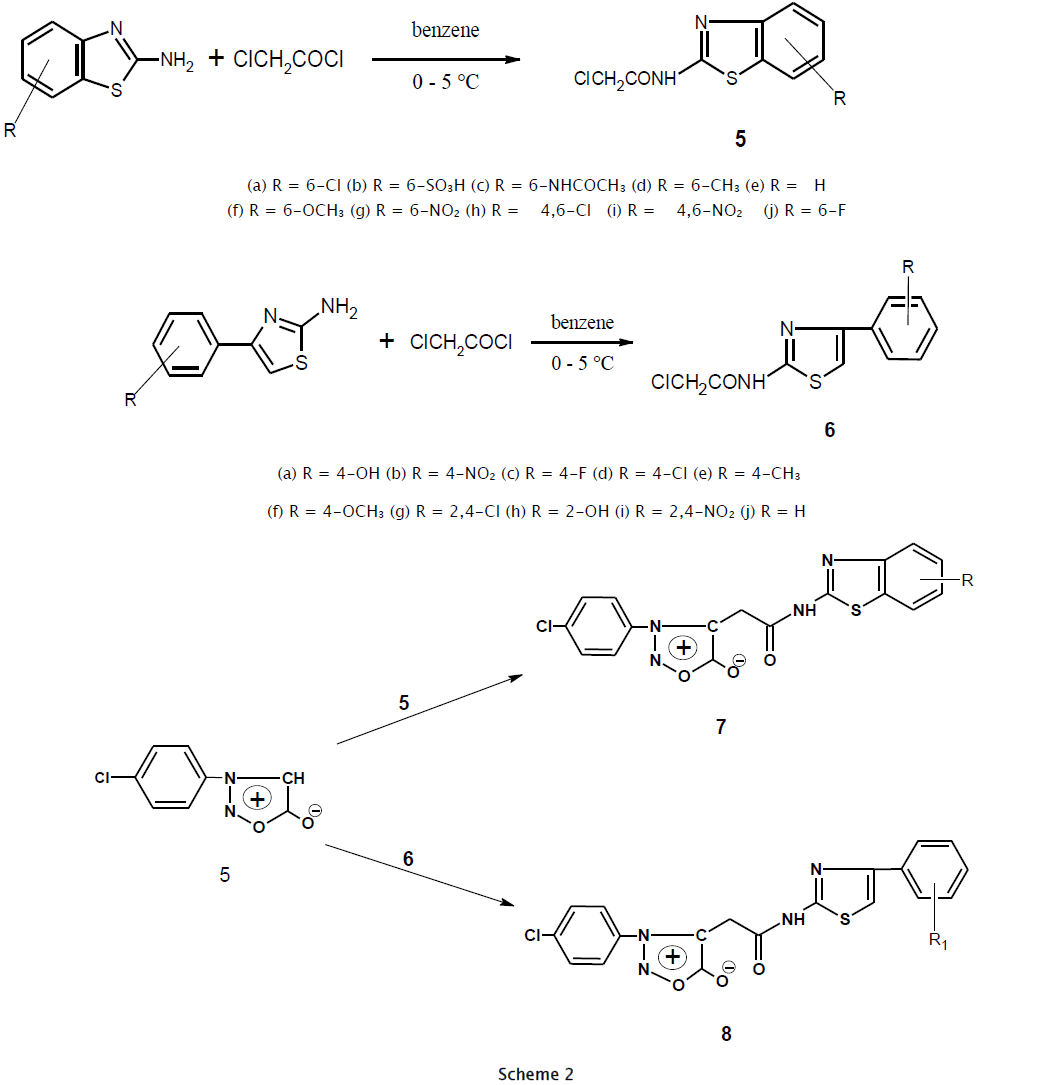
The effectiveness of mesoionic compounds as a chemotherapeutic agent is well established [1,2,3,4] and their chemistry has been studied. A literature survey revealed that sydnones display potent biological activity [5,6,7,8,9]. Feprosidnine (sydnophen) is a CNS-stimulant drug in which the sydnone imine group is found as sub-structure [10]. Sydnones occupy a unique place among mesoionic compounds [11]. The sydnone ring displays electron-withdrawing character at N3 and electron-donating properties at C4. The hydrogen atom of sydnone ring undergoes substitution with variety of electrophiles [12]. Although various 4-substituted sydnones have been reported over last seven decades, no method has been reported to synthesize acetamide derivatives of sydnones. The thiazole ring found to be a part of many natural products. We now report the synthesis of new sydnones bearing acetamide groups at position 4 in high yields. 3-(4-Chlorophenyl)sydnone (4) was prepared in improved yields according to the literature procedure [20]. 2-Chloro-N-(substituted-1,3-benzothiazol-2-yl)acetamide (5) and 2-chloro-N-[4-(aryl)-1,3-thiazol-2-yl]acetamide (6) were synthesized by procedures reported in the literature [13,14,15]. Reaction of 3-(4-chlorophenyl)sydnone (4) with 5 and 6 in the presence of triethylamine gave the C-substituted sydnones 7 and 8 in good yields (70-85 %). Since abstraction of the C4 hydrogen of sydnones usually requires strong bases such as Grignard or organolithium reagents [16,17,18], it is likely that the activated halides in our case are first attacked by the C4 atom followed by removal of the proton by triethylamine. There are no reported cases of alkylation of sydnones by simple alkyl halides in the presence of triethylamine. All the compounds gave satisfactory combustan analyses and were characterized by elemental analysis, IR, 1H NMR and 13C NMR data. Spectral data for the new compounds are consistent with the assigned structures.
Mps were recorded using an electrothermal melting point apparatus and are uncorrected. TLC was performed on E-Merck pre-coated 60 F254 plates and the spots were rendered visible by exposing the plates to UV light. CHN analyses were carried out on a Carlo Erba 1108. The infrared spectra were scanned on a Nicolet-400D spectrometer. NMR spectra were recorded on Brucker Avance II NMR spectrometer. Chemical shifts (δ) refer to internal tetramethylsilane.
Ethyl N-(4-Chlorophenyl)glycinate (1). 4-Chloroaniline (1.40 g, 0.01 mol), ethyl chloroacetate (1.06 mL, 0.01 mol) and anhydrous sodium acetate (1.64 g, 0.02 mol) were refluxed in ethanol (10 mL) for 5 hours. The mixture was diluted with 10 mL of water and kept in refrigerator over night. Recrystallization from ethanol afforded 1.86 g. (81%), mp. 115-117 °C, lit.19 mp. 94 °C. IR (KBr): 3328, 2951, 2933, 2887, 1757, 1072; 1H NMR (400 MHz, DMSO-d6): δ 1.21 (t, 3H, COOCH2CH3), 3.76 (s, 1H, NH), 4.29 (s, 2H, CH2), 4.54 (q, 2H, COOCH2CH3), 6.83-7.21 (m, 4H, Ar-H); 13C NMR (40 MHz, DMSO-d6): δ 14.67, 44.78, 61.29, 115.12, 123.22, 128.96, 146.26, 172.11
N-(4-Chlorophenyl)glycine (2). Ethyl N-(4-chlorophenyl)glycinate (2.13 g, 0.01 mol) and sodium hydroxide (0.6 g, 0.015 mol) in solution of aqueous ethanol (18 mL:4 mL) was heated for 0.5 hour. It was then cooled in ice and acidified with conc. hydrochloric acid till complete precipitation. The crystalline white product obtained was recrystallized from ethanol to yield 1.29 g. (70 %), mp. 145-147 °C. lit.19 mp. 144 °C. IR (KBr): 3323, 3278-2521, 2954, 2937, 2881, 1705, 1067; 1H NMR (400 MHz, DMSO-d6): δ 4.33 (s, 2H, CH2), 6.44 (s, 1H, COOH), 6.52 (s, 1H, NH), 6.88-7.23 (m, 4H, Ar-H); 13C NMR (40 MHz, DMSO-d6): δ 44.98, 114.32, 123.26, 129.10, 146.07, 172.18.
(4-Chlorophenyl)-N-nitrosoglycine (3). To a solution of N-(4-chlorophenyl)glycine (1.86 g, 0.01 mol) in conc. hydrochloric acid (20 mL), a solution of sodium nitrite (0.69 g, 0.01 mol) in water (5 mL) was added dropwise at 0-5 °C with stirring for 2 hour. The product obtained was collected and recrystallized from ethanol to yield 1.67 g. (78%) of pale yellow solid, mp. 104-106 °C. lit.20 mp. 113 °C. IR (KBr): 3257-2526, 2925, 2853, 1712, 1571, 1328, 1065; 1H NMR (400 MHz, DMSO-d6): δ 5.02 (s, 2H, CH2), 6.93-7.48 (m, 4H, Ar- H), 11.56 (s, 1H, COOH); 13C NMR (40 MHz, DMSO-d6): δ 49.43, 120.78, 128.31, 130.46, 138.89, 168.24.
3-(4-Chlorophenyl)sydnone (4). The mixture of (4-chlorophenyl)-N-nitrosoglycine (2.70 g, 0.0126 mol) and acetic anhydride (15 mL) was stirred at room temperature for 12 hours in a dark. The solution was poured slowly into cold water with vigorous stirring. The pH of the solution was adjusted to 7.0 with 10 % sodium bicarbonate solution. The crude sydnone obtained was washed well with water and dried. Recrystallization from ethanol afforded yield 2.14 g. (87 %) of colorless solid. mp. 140-145 °C. lit. [21] mp. 110-112 °C. IR (KBr): 3178, 1750, 1056; 1H NMR (400 MHz, DMSO-d6): δ 7.21 (s, 1H, sydnone), 7.52-8.16 (m, 4H, Ar-H); 13C NMR (40 MHz, DMSOd6): δ 124.12, 128.55, 130.63, 135.22, 140.92, 169.19
General Procedure for synthesis of N-(Substituted-1,3-benzothiazol-2-yl) and N-(4-Substituted-1,3-thiazol-2-yl)-2-chloro acetamide 5a-j [9,10,11]. The substituted benzothiazole or thiazole (0.01 mol) was dissolved in benzene (25 mL) and stirred for 30 minute at 0-5 0C. Chloroacetyl chloride (0.8 mL, 0.01 mol) was then added dropwise over 2 hrs. The mixture was heated at reflux for 2 hrs. Removal of solvent by distillation gave crude solid which was recrystallized from methanol.
4-{2-[(6-Chloro-1,3-benzothiazol-2-yl)amino]-2-oxoethyl}-3-(4-chlorophenyl) sydnone (7a). To a solution of 3-(4- chlorophenyl)sydnone (1.96 g, 0.01 mol) in acetone (25mL) was added 2-chloro-N-(6-chloro-1,3-benzothiazol-2-yl)acetamide (4.21 g, 0.01 mol) and 2-3 drops of triethylamine. After 6 hrs of reflux, the reaction mixture was cooled and poured into ice. The solid product obtained was collected and dried. Recrystallization from methanol yielded 3.27 g. (78 %) of colorless solid.
The analogues 7b-e were prepared and purified as in the procedure given for 7a.
The analogues 8a-e were prepared and purified as in the procedure given for 7a
The IR spectrum of 5 showed absorption bands at 1750 cm-1 for C=O stretching of sydnone ring. The IR spectra of these products generally showed absorption bands at 1745-1778 cm-1 for C=O group of the sydnone ring and the peaks at 1664-1693 cm- 1 are attributed to the C=O of the amide linkage and the 663-693 cm-1 bands are due to C-S-C stretching of thiazole and benzothiazole ring. Furthermore, the 1H NMR spectra of sydnones 7 and 8 showed signals in the range of δ 3.42-3.63 for CH2 group and δ 8.95-11.75 for CONH group and the absence of the characteristic sydnone C-H peak at δ 7.21. Furthermore, the 13C NMR spectra exhibited confirmatory signals for the carbonyl carbon of sydnone ring around δ 167.00 and the carbon atom at position 5 of the thiazole ring showed signal about δ 110.00.
From the present work, compounds 7(a-j) and 8(a-j) were synthesized by using different substituted benzothiazoles 5(a-j) and different substituted thaizole 6(a-j) condensation with sydnone moieties. All the compounds synthesized in scheme I & II are recrystallized from ethanol afforded good yield.
The authors express their thanks and gratefully to the SAIF, Chandigarh for carrying out elemental analyses and NMR spectra. The authors are thankful to the Principal, Silvassa Institute of Higher Learning, Silvassa for providing the research facilities. The authors are also thankful to the Department of Chemistry, VNSG University, Surat for recording the IR spectra and for providing laboratory facilities. The authors express their sincere gratitude to the Atul Limited, Valsad for providing some chemicals.