ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
P. Indira1, S. Kondala Rao2 and K.V.R. Murthy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Eu³Ã¢Âº doped rare earth ortho phosphates (rare earths = La, Y) have been prepared by solid state diffusion reaction method. The required starting materials were weighed and grounded by using mortar and pestle, fired at 1200oC for 3 hours in a muffle furnace. Photoluminescence (PL) of the LaYPO₄ phosphor doped with Eu (0.5%) and Eu (0.5%, 1.0%, 1.5%, 2.0%) : Gd 0.5% keeping Gd concentration constant are studied and presented in this paper. The produced phosphor materials were analyzed using XRD, PL, FTIR and SEM techniques. We have studied the effect of dopants on the photoluminescence of LaYPO₄ phosphor under the excitation of 254nm wave length. PL properties of the samples are recorded using Spectrofluorophotometer at room temperature. PL emission of LaYPO₄ doped with Eu (0.5%) phosphor shows peaks at 534, 586, 590, 597,612, 621 and 631 nm with good intensity alog with blue green region with less intensity. PL emission of Eu (0.5%, 1.0%, 1.5%, 2.0%) : Gd 0.5% shows peaks at same wavelength of LaYPO₄ doped with Eu (0.5%) As the Eu concentration increases the PL intensity also increases up to Eu concentration 1.5%. For Eu concentration 2.0% the intensity slightly decreased.
Keywords |
| Photoluminescence (PL), Solid state reaction method (SSR), Mixed rare earth ortho phosphates, Nano phosphors |
INTRODUCTION |
| Nano structure luminescent materials with controlled chemical composition, crystal phase, structure, morphology and particle size have been extensively investigated during the past few decades because of their high surface/volume ratio and the special quantum confinement effect [1,2]. Nano luminescent materials can show remarkable tunable properties and play an important role as active components in the preparation of optical optoelectronic devises [3-7]. Herein, the fabrication of nano materials will well-controlled dimensionality, morphologies, phase purity and desired properties remains one of the most challenging issues[8]. One simple method to control the particle size and morphology is solid state reaction diffusion method which is extensively employed in the synthesis of rare earth ions activated inorganic compounds such as yttrium van date, lanthanum phosphate and lanthanum yttrium phosphate. Because of their excellent luminescent properties rare earth phosphates have been extensively applied as phosphors for laser hosts, heat resistant materials and moisture sensors whose crystal structure and synthesis technology have been studied long time ago [9]. For example LaPOâÃâÃâ:Ce,Tb phosphors have been used as green emission component in tri-chromatic luminescent lamp [10]. Presently it is important to synthesis rare earth phosphate phosphors with good morphology, composition and size. Ever since Y.S. Patil et.al. has fabricated LaPOâÃâÃâ:Eu and LaPOâÃâÃâ:Tb nanocrystals by standard solid state reaction method, lots of work have been focused under the study of rare earth phosphate nano crystals [11]. Therefore it is very interesting that what will happen where rare earth ions are introduced into one REPOâÃâÃâ systems with phosphates. In this paper we have investigated and discussed the results on the crystal phase, structure, PL, microstructure (morphology and particle size) of the mixed phosphates prepared by standard solid state diffusion reaction method. At the same time Eu³Ã¢ÃÂú ions and Gd³Ã¢ÃÂú ions have been doped in the mixed rare earth phosphates in order to examine the influence of the hosts on the luminescence of Eu³Ã¢ÃÂú and Gd³Ã¢ÃÂú whose photo luminescent behaviors are studied in detail. |
SYNTHESIS OF MIXED PHOSPHATES |
| The starting materials Lanthanum oxide (LaâÃâÃâOâÃâÃÆ), yttrium oxide (YâÃâÃâOâÃâÃÆ), Europium oxide (EuâÃâÃâOâÃâÃÆ) and Gadolinium oxide (GdâÃâÃâOâÃâÃÆ) of high purity (99.9%) chemicals were used as starting materials to prepare LaYPOâÃâÃâ and Eu, Gd doped phosphor. Lanthanum oxide (La âÃâÃâOâÃâÃÆ), Yttrium oxide in stoichiometric proportions is weighed and ground into a fine power using agate mortar and pestle. The grounded sample were placed in an alumina crucible and fired at 1200°C for 3 hours in muffle furnace with a heating rate of 5°C /min. The sample is allowed to cool room temperature in the same furnace for about 20 hours. Rare earth ions Eu, Gd and Eu:Gd were doped and co doped keeping Gd concentration at 0.5% and changing Eu concentration from 0.5%, 1.0%, 1.5% and 2.0%. Spectrofluorophotometer (SHIMADZU, RF-5301 PC) was used for PL studies. |
PHYSICAL CHARTERIZATION |
| The X-ray powder diffraction (XRD) pattern of samples is performed on a Rigaku-D/max 2500 using Cu Kα radiation. The microstructures of the sample were studied using scanning electron microscopy (SEM) (XL 30 CP Philips). The particle size distribution histogram recorded and particle size was measured using laser based system Malvern instrument U.K. All the spectra were recorded at room temperature. |
RESULTS AND DISCUSSIONS |
| Crystal phase and microstructure of mixed rare earth phosphate: |
| Li et.al, have studied the crystal phase structure of the mixed rare earth phosphates indicating the pure LaPOâÃâÃâ and YPOâÃâÃâ crystallize in monoclinic phase and tetragonal phase respectively, while the mixed phosphate LaYPOâÃâÃâ belongs to La0.5Y0.5 POâÃâÃâ belong to the hexagonal phase [12]. The XRD pattern of LaYPOâÃâÃâ doped with Eu and doped with Eu 1.5%, Gd 0.5% are shown in figure 1. From the XRD pattern it was the found that the prominent phase formed is LaYPOâÃâÃâ, after diffraction peaks were well indexed based on JCPDS no. 32-0493 indicating monoclinic phase of monazite structure. The main peak was found around 29.127° corresponding to a d-value of about 3.10A° followed by other less intense peaks corresponds to the monoclinic system of crystal structures of Lanthanum Yttrium phosphate [13]. All diffraction patterns were obtained using Cu Kα radiation (λ= 1.54060 A°). Measurements were made from 2θ=18° to 60° with steps of 0.008356°. |
| The crystallite size was calculated using the Scherrer equation D=k λ/β cos θ. Where the constant (0.9) λ the wave length of X-rays (1.54060 A°), β the ful-width at half maxima (FWHM) and θ the Bragg angle of the XRD peak. The calculated average crystallite size of LaYPOâÃâÃâ: Eu 0.5% is ~ 39 nm. |
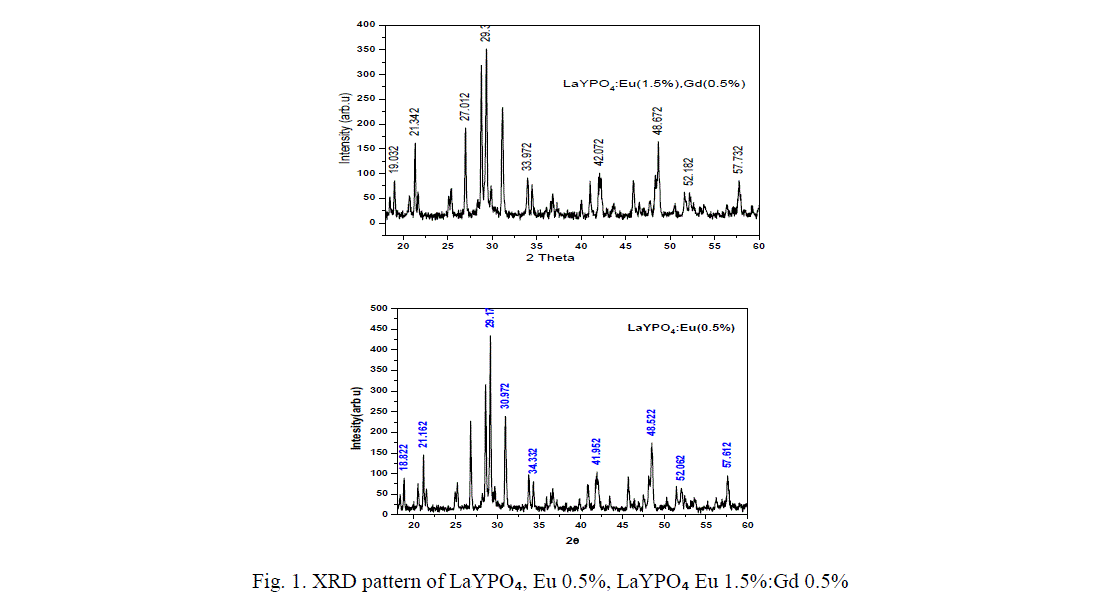 |
| From figure 1 it is found that diffraction peaks are slightly shifted when co-dopent added to LaYPO4:Eu(0.5%):.The calculated average crystallite size of Eu (0.5%) doped is around 39 nm, Gd (0.5%) : Eu (1.5%) doped is ~ 46 nm. Introduction of Gd ions in LaYPO4:Eu(0.5%): increases the crystallite size by 15%. |
SEM STUDY |
| Characterizing particles of feature size for nano crystals and nano structures is done routinely using scanning electron microscope. The main advantage of SEM is that they can be used to study the morphology of prepared nano particles and nano composites. Direct size measurements obtained from images are often used in conjunction with other measurements such powder, X-ray diffraction (XRD). Figure 2 a and b shows the SEM micrographs of LaYPỎ̢̉, Eu 0.5%, LaYPỎ̢̉ Eu 1.5%:Gd 0.5% phosphors. Direct size measurements obtained from images and average particle diameter is observed. From the Scanning Electron Micrograph of LaYPỎ̢̉, Eu 0.5%, LaYPỎ̢̉ Eu 1.5%:Gd 0.5% phosphors it is found the particles are irregular in shape with various sizes from of submicron to few micros and also clusters are found. |
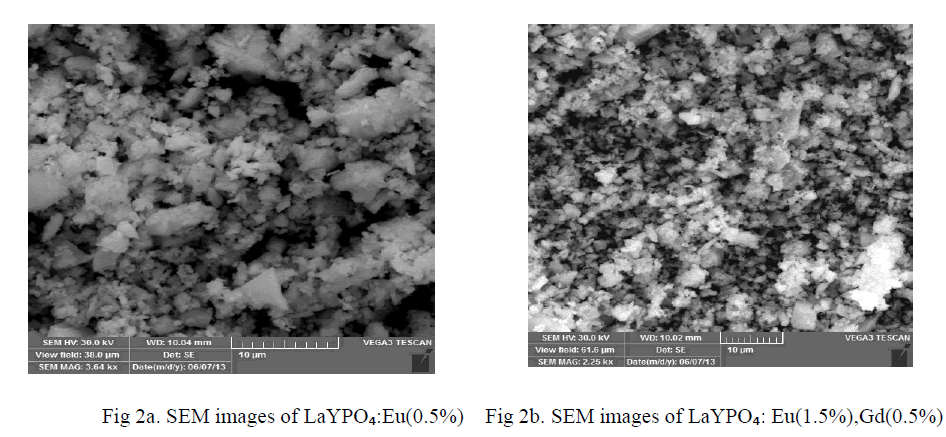 |
PHOTO LUMINESCENCE STUDY |
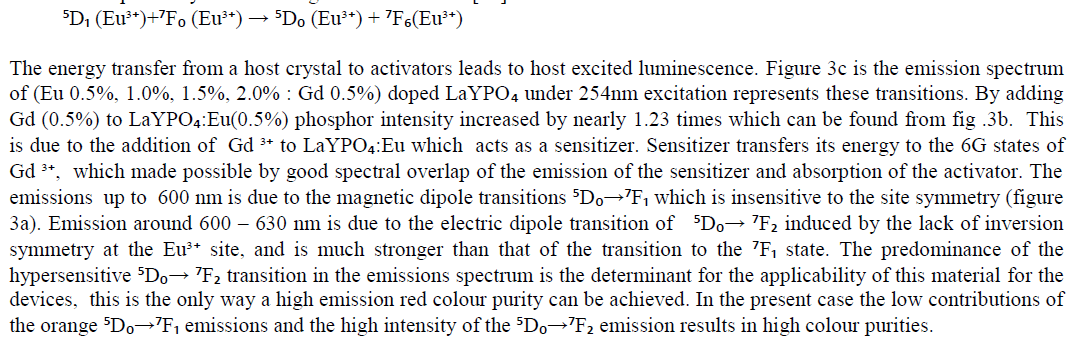
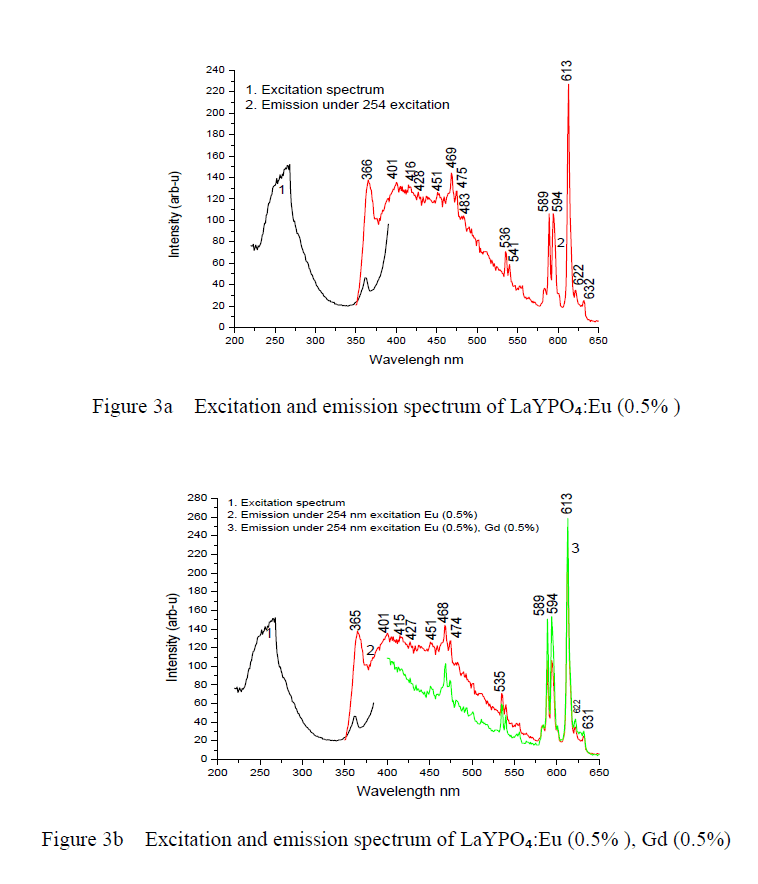
| Optical spectroscopic measurements are quite important to determine colour purity and quantum efficiency. The photoluminescent properties under UV excitation of the obtained phosphates are presented in figure 3a, 3b and 3c. The excitation spectrum of LaYPOâÃâÃâ:Eu³Ã¢ÃÂú (0.5%) and Eu3+ doped and Gd3+ co doped represents a broad and intense band at maximum at 254 nm, related a ligand-metal charge transfer between phosphate groups and Eu³Ã¢ÃÂú ions. Figure 3a the emission spectrum of LaYPOâÃâÃâ:Eu³Ã¢ÃÂú. The luminescence spectra of Eu³Ã¢ÃÂú have strong dependence under the concentration. This is because at higher concentrations, the higher emitting levels, âÃÂõDâÃâàof Eu³Ã¢ÃÂú, transfer their energies to neighboring ions of the same species by the following cross relaxations [14]. |
 |
 |
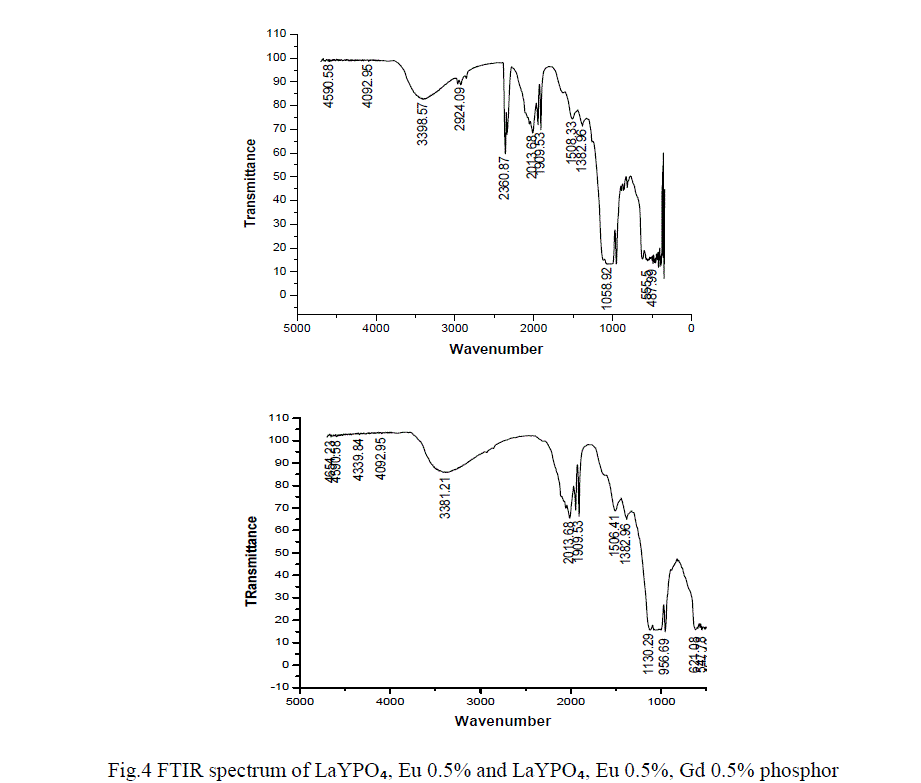 |
FTIR ANALYSIS |
| In order to determine the atomic bonds in a molecule FTIR analysis was carried out. Figure 4 shows the FTIR spectrum of LaYPỎ̢̉, Eu 0.5% and LaYPỎ̢̉, Eu 0.5%, Gd 0.5% phosphor. From FTIR spectrum it is observed that the peak at 3398 cm -1 is assigned to inter molecular OH sharp band of H2O. The specimen might have absorbed moisture from the atmosphere. The absorption peaks at 2360 and 1058 are assigned to stretching and bending vibrations respectively. |
 |
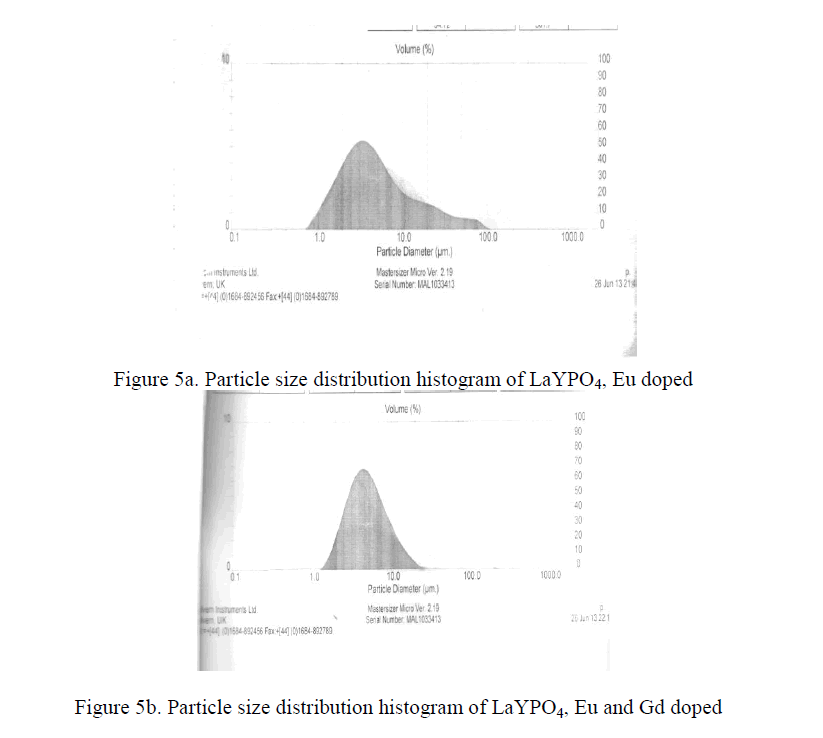
PARTICLE SIZE |
| Practical phosphors must be prepared so that they can form a dense, pinhole coating on a substrate. This property is determine mainly by particle size distribution. The optimum coating thickness is roughly proportional to its mean particle size i.e. the smaller the particle size, the thinner the coating can be. Therefore a small particle size advantages for expensive phosphors. The Y2O3 : Eu3+ phosphors and some aluminate phosphors like Ba Mg2 Al16 O27 : Eu 2+ can be made highly efficient at mean particle diameter of about 3 micrometers [15-18]. The LaYPO4 doped with Eu 0.5% having particle size 4.2 micrometers and LaYPO4 doped with Eu 0.5% and Gd 0.5% having particle size 4.5 micrometers. From XRD studies the average crystallite size is around 39 nm. As such many molecular particles agglomerate and form as a crystallite and many crystallite together became a particle. |
 |
CONCLSIONS |
| Pure LaYPO4 phosphor doped with Eu(0.5%) and Eu co-doped with Gd in LaYPO4 phosphors were synthesized via high temperature solid state diffusion reaction. PL emission intensity of LaYPO4 doped with Eu(0.5%) recorded.PL emission intensity of LaYPO4:Eu(0.5%) co-doped with Gd(0.5%) recorded and observed that peak intensity increased because Gd acts like sensitizer and also observed that Eu concentration increases peak intensity increased up-to Eu (1.5%). For Eu(2.0%) peak intensity slightly decreased this is due to quenching effect. From previous studies halophosphate phosphors having minimum particle size is around 8μm[16] .But LaYPO4 doped with Eu and Eu:Gd have particle size is 4.2 μm and 4.5 μm respectively. Particle size is low when compared with general phosphate phosphors. So this phosphor is a good candidate for fluorescent lighting, display devices , X-ray monitoring and imaging, scintillators and biomedical imaging applications. |
References |
|