ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
K. Manjula Rani1, S.P. Rajendran2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Pyrazolo derivatives have been the basis of numerous drugs and dyes used in industries, hitherto of its possessing analgesic and antipyretic properties in addition to its base unit exhibiting dyeing characteristics. No doubt, owing to their synthetic utility in many products, interest in developing a new procedure becomes enviable. Quite a large number of medicinal plants in Indian subcontinent contain heterocyclic components in roots, stem and shoot system. Also a good number of the Pyrazolo derivatives are found in seeds, for example water melon seeds found to contain Pyrazolo in the concentration range of 250-410 μg/l on a specific variety. As for the bio synthetic activity is concerned, most plants have properties relating to anti-inflammatory, diagnostic acid, antibacterial, hypoglycemic, sedative-hypnotic etc. Herein, this paper deals with the synthesis and characterization of Pyrazolo derivative namely pyrazolidino [3, 4-b] quinoline by IR and NMR techniques. The data reveals the modified mode of synthesis from an intermediate stage up to the finalized product pertaining to the structure identified base as Pyrazolo quinoline.
Keywords |
| Pyrazolo, quinoline, anti-inflammatory, analgesic, IR, NMR. |
INTRODUCTION |
| 1 H – Pyrazolo [3,4 – b] quinolines are of interest in a number of diverse contexts for example as antiviral agents1 in particular as inhibitors of Herpex simplex virus type2,3, replication4, as activators of caspases and inducers of apoptosis, as antimicrobial agents5,6 as Parasiticidic agents as antimalarials , in photoluminescence7 and electroluminescence8 studies, and in electroluminescent devices9. The use of 1 H – Pyrazolo[3,4-b] quinoline derivatives as optical brighteners has been long known10 and interest in them as dyestuffs11,12 and as colorants within polymer13, continues to the present. In continuation of this work, we report herein a synthesis of pyrazolo [3, 4-b] quinoline derivatives utilizing the 2-chloro-3-formyl quinoline and 3-cyano-4-phenyl-2-quinoline (7) as starting material under scheme 1&2. |
EXPERIMENTAL SECTION |
| General Experimental Procedures |
| All melting points were determined by using Mettler “FP5” instrument and were uncorrected. IR spectra were recorded on Perkin Elmer – 597 Spectrometer as KBr disc and the absorption frequencies are expressed in reciprocal centimeters (cm-1). 1H-NMR Spectra were recorded in CDCl3 or DMSO –d6 on FX90 QFT NMR Spectrophotometer using TMS as an internal standard and chemical shifts are expressed as δ ppm units. The homogeneity of all compounds synthesized were checked by TLC and purified by column chromatography. Characterization data of the various compounds prepared are given in Tables - 2 and Table - 3. |
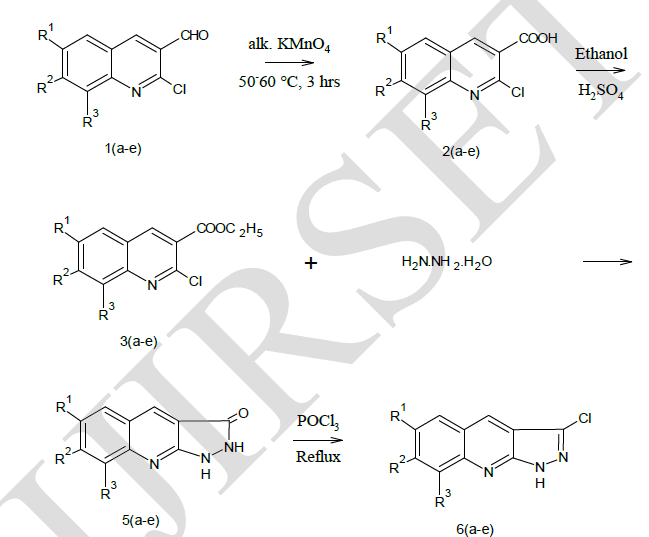
| Preparation of 2-chloro-3-formy quinolines (1a-e) |
| Dimethyl formamide (2.5 mol.equ.) was cooled to 0ïÃâðC in a flask equipped with a dropping funnel. Phosphoryl chloride (7 mole/equiv.) was added drop wise with stirring. To this, acetanilide (1 mole/equ) was added and after 5 minutes the solution was heated under reflux for appropriate time (6 – 16.5 hrs). The reaction mixture was then poured into crushed ice and stirred for 30 minutes at 0 – 10ïÃâðC. The solid was filtered and dried. |
| Preparation of 2-chloro quinoline-3-carboxylic acid 2(a-e) |
| To 2-chloro-3-formy quinoline (0.01 mole) in water (100 ml), alkaline KMnO4 (0.15 mole) and 10% NaOH was added slowly. The flask was surrounded by hot water and stirring continued for 4 hours. The reaction mixture was filtered and the filtrate on neutralization with aqueous HCl (1:1) gave the acid. The acid was purified by dissolving in sodium bicarbonate (10%) solution and neutralized with dilute HCl. The obtained acid was filtered, washed and dried. |
| Preparation of Ethyl-2-chloro quinoline-3-carboxylate 3(a-e) |
| A mixture of Preparation of 2-chloroquinoline-3-carboxylic acid (0.005M), 100 ml of absolute ethanol and 2 ml of concentrated sulphuric acid was refluxed for seven hours on preheated water bath. After distilling off the excess alcohol, it was poured into water. The solid obtained was filtered and recrystallized from a suitable solvent. |
| Preparation of Pyrazolo [3, 4-b] quinoline 5(a-e) |
| To 0.5g of compound(3) (0.002 mole), 5 ml of 80% hydrazine hydrate in absolute ethanol and few drops of triethylamine was added and refluxed on water bath for 15 hours. After completion of the reaction, the excess hydrazine hydrate and alcohol were distilled off under reduced pressure and poured into water. the solid obtained was filtered and dried. |
| Preparation of Pyrazolidino [3,4-b] quinoline 6(a-e) |
| Compound (5) (0.500g) was taken in a round bottomed flask and 4 ml of Phosphoryl chloride and 2 drops of N, N – dimethylaniline were added and refluxed on a water bath for five hours . The reaction mixture after cooling was poured in to crushed ice, neutralized with ammonium hydroxide solution and extracted with chloroform. The solvent was evaporated and the solid (0.356 g) obtained was dried and recrystallized from ethanol. |
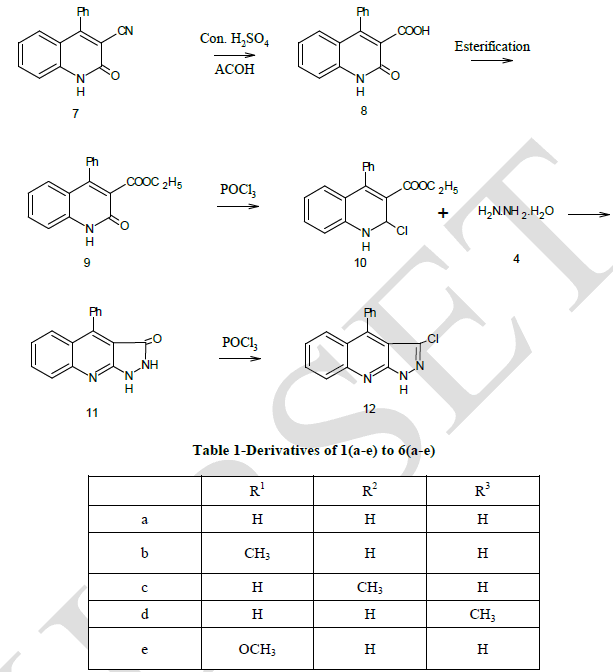
| Preparation of 3-cyano-4-phenyl-2-quinoline (7) |
| 2-amino benzophenone (1.97g, 1 mole) was mixed with ethyl cyanoacetate (1.12g, 1 mole) and heated in an oil bath at 180-185 C for 6 hrs. After cooling the resulting solid was washed with ethyl acetate and recrystallized from ethanol. |
| Preparation of 2 – oxo-4-phenyl quinoine-3-carboxyllic acid (8) |
| Compound (7) (2.46g, 0.01 mole) was hydrolyzed by refluxing it with a mixture of concentrated sulphuric acid and acetic acid (80:20) for 2 hrs. The reaction mixture was cooled and poured into ice water. The solid separated was filtered and washed with water. The compound (8) was purified by dissolving it in sodium bicarbonate solution (10%) and neutralizing it with dilute HCl (10%). |
| Preparation of ethyl-2-oxo-4-phenyl quinoline-3-carboxylate (9) |
| Compound (8) (1.5 g) and 50 ml of absolute ethanol and 2 ml of concentrated sulphuric acid was refluxed for 7 hrs on a preheated water bath. After distilling off the excess alcohol, it was poured into water. The solid obtained was filtered and recrystallized. |
| Preparation of Ethyl-2-choloro-4-phenyl quinoline-3-carboxylate (10) |
| To 2 g of compound (9), 14 ml of Phosphoryl chloride and 1 ml of N,N-dimethyl aniline were added and refluxed on a preheated water bath for 3 hrs. The reaction mixture after cooling was poured into crushed ice, neutralized with ammonium hydroxide solution. The precipitate formed was filtered and dried. |
| Preparation of 4-phenyl pyrazolidino [3, 4-b] quinoline (11) |
| To 0.500 g of compound (10) 5 ml of 80% hydrazine hydrate, 10 ml of ethanol and few drops of triethylamine were added and refluxed on a water bath, for 15 hrs. After distilling off the excess hydrazine hydrate and alcohol under reduced pressure the reaction mixture was poured into water. The solid obtained was filtered and dried. |
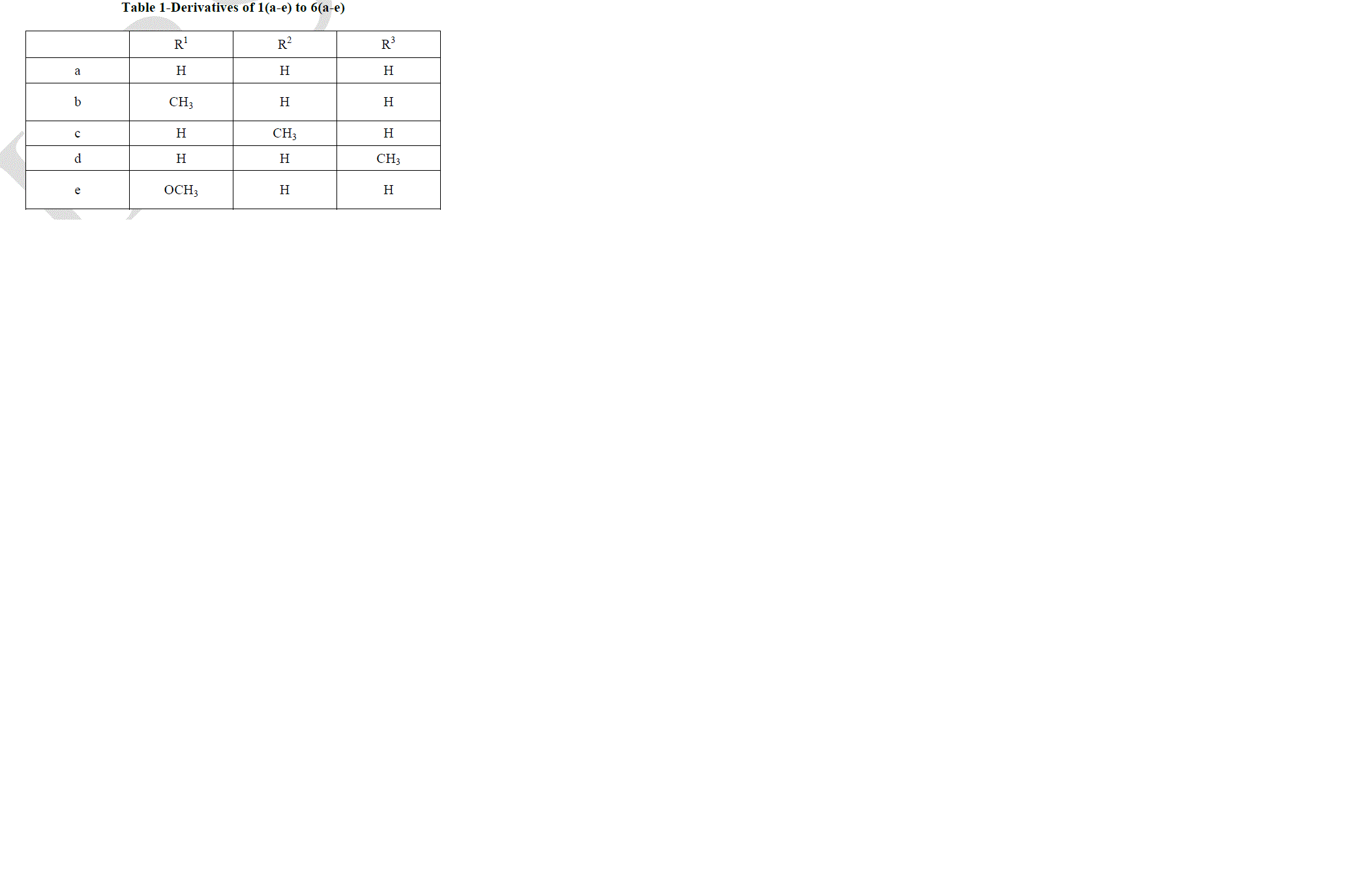 |
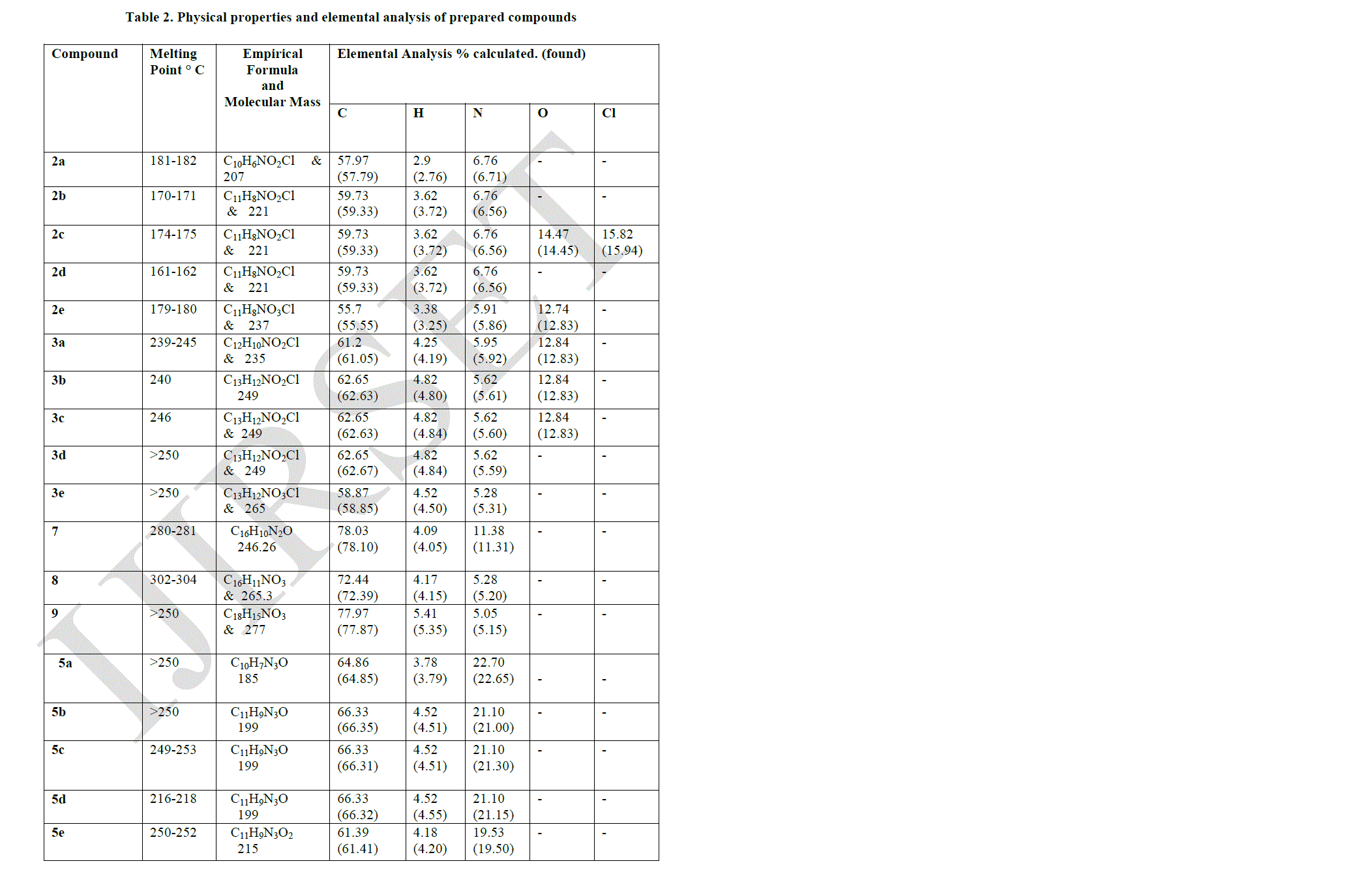 |
RESULTS AND DISCUSSION |
| Otto Meth-Cohn et al14 synthesized the precursor 2 – chloro-3-formyl quinolines form various substituted acetanilide by the action of Vilsmeir’s reagent (Dimethyl formamide / Phosphoryl Chloride). The reaction sequence involves the successive conversion of acetanilide (A) into a imidoyl chloride and then into N- ( α-chloro vinyl) anilines 55, which were diformylated at its β-position to yield the cyclised product, chloro quinoline aldehyde.1 |
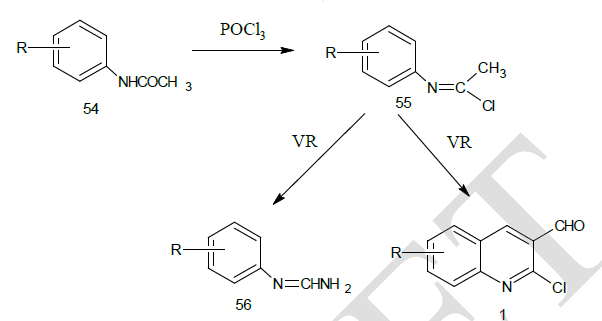 |
| From the above reaction, it was observed that with electron donating groups on phenyl ring yielded the required chloro quinoline aldehydes while these with electron withdrawing groups gave the uncyclised enamine 56. |
| Mechanism of Conversion19 |
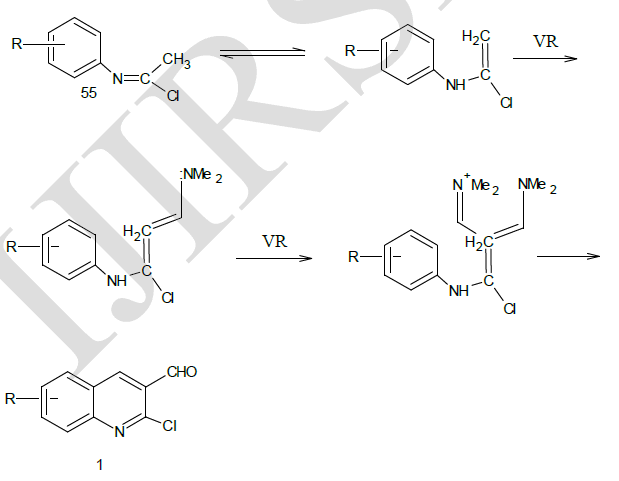 |
| According to the above procedure, we prepared 6-methyl, 7–methyl, 8–methyl and 6-methoxy derivatives of 2-chloro- 3-formyl quinoline. |
| Scheme 1 |
| Pyrazolo [3, 4-b] quinoline derivatives 6(a-e) as given in Table-1 were prepared from 2-chloro-3-formyl quinolines 1(a-e) via oxidation, esterification and fusion. The structure of 6(a-e) was assigned on the basis of its elemental analysis and spectral analysis (Table-2 &Table-3).Further the structure of 5c was supported by 1H-NMR data. |
 |
| Scheme 2 |
| Compound (7) was first converted to 4–phenyl-2- quinoline -3- carboxylic acid (8) by using concentrated sulphuric acid and acetic acid. The acid (8) undergoes esterification in presence of ethanol and sulphuric acid gave the corresponding ester which refluxing with POCl3 in presence of N, N – dimethyl aniline gave compound (10). Compound (10) undergoes fusion with compound (4) gave the desired Pyrazolo quinoline compound (11). The structure of the isolated product was confirmed by elemental analysis given in Table-2 and spectral analysis given in Table-3. |
 |
CONCLUSION |
| The present investigation reports that the synthesis of Pyrazolo [3, 4-b] quinoline derivates 6(a-e) were prepared from 2-chloro-3-formyl quinolines 1(a-e) via oxidation, esterification and fusion and 4-phenyl pyrazolidino [3, 4-b] quinoline (11) from 3-cyano-4-phenyl-2-quinoline (7). And also the structures of synthesised materials were confirmed by spectral analysis. |
References |
|