ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Ganesh R. Mhaske1, ShivdasS. Bajod2, DamodharM. Ambhore2 and Sharad N. Shelke1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Novel 1, 5-benzothiazepine derivatives were synthesized and characterized by spectral studies. The newly synthesized compounds (4a–g) were screened for in vivo anti-inflammatory activity at a dose of 10 mg/kg BW. Among those tested, compounds 4c, 4d and 4g exhibited significant anti-inflammatory activity in models of acute inflammation such as rat paw edema, while compounds 4c and 4g showed considerable activity compared with diclofenac as a standard drug.
Keywords |
| Benzothiazepine, chalcone, pyrazole, anti-inflammatory activity |
INTRODUCTION |
| Organic synthetic chemistry is now a fast growing research field in chemistry. Among the various organic compounds, heterocyclic compounds have been associated with various biologically activities. Due to bioactivity connected with heterocycle and ease of preparation, a number of researchers are takings keen interested into the study of this. |
| N- and S- containing heterocycle, such as thiazepine and its derivatives, exhibit a broad spectrum of biological activity1,2. Thiazepine fused with a benzene ring is known as benzothiazepine, and it is associated with antibacterial, antifungal3, antimicrobial4, anticonvulsant5, and anti-breast cancer activity6, acting as a central nervous system depressant7. |
| Pyrimido[4,5-b]-1,4-Benzothiazepine draws considerable attention because it is used as an inhibitor of the epidermal growth receptor tyrosine kinase8 and is applicable for stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex9. In the last decade, a series of monocyclic thiazepine inhibitors of the interleukin-1ïÃÂâ converting enzyme (ICE) were synthesized10 and also exhibited neuroprotective properties11. |
| As 1,5-benzothiazepine plays an important role in the pharmacological and medicinal field, various researchers are interested in its synthesis12 and characteristics13, 14. Recently, synthesis and a biological evaluation of thiazepine from chalcone and 2-aminoethanethiol has been investigated15, and a written survey revealed that different synthetic routes of thiazepine had been reported16, 17. |
| Encouraged by the significance of benzothiazepine cited in literature and the movement of our work in the bioorganic field18–20, we have studied its anti-inflammatory activity. In this current investigation, we report the synthesis, biological evaluation and preliminary structure activity relationship (SAR) of benzothiazepine derivatives. |
RESULT AND DISCUSSION |
| Chemistry |
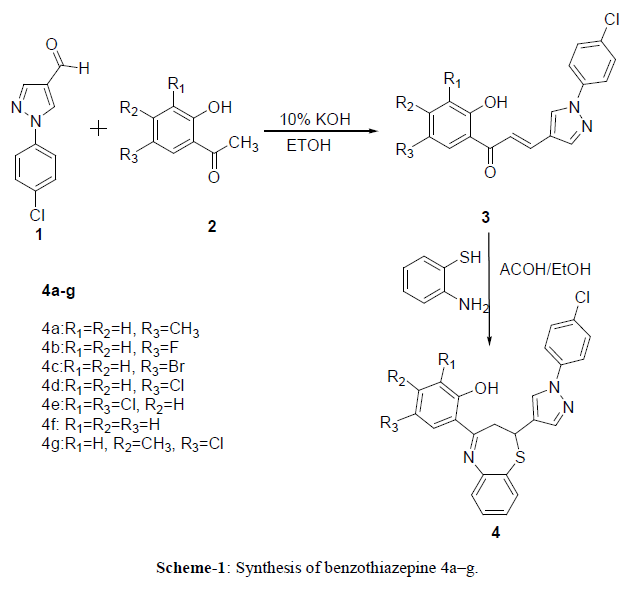
| The synthetic route used to synthesize the target compound 4a–g is outlined in Scheme 1. 1-(4-chlorophenyl)-1Hpyrazole- 4-carbaldehyde 1 was prepared by Vilsemeier-Haack reaction of the acetaldehyde N-(4- chlorophenyl)hydrazone21. |
| Chalcones 3a–g were prepared by the Claisen–Schmidt condensation reaction of 1-(4-chlorophenyl)-1H-pyrazole- 4-carbaldehyde 1 and variously substituted o-hydroxyacetophenones2 in the basic medium. The target compounds 4a– g were synthesized by Michael addition of o-amino thiophenol to chalcones3 in acetic acid/ethanol. |
| The purity of the synthesized compounds was checked by TLC. The proposed structure of the synthesized compound has been confirmed by spectral studies. |
| In general, three thiazepine protons of known benzothiazepines4a–g showed similar patterns of signals in 1H NMR. They displayed doublet of doublet (dd) for two protons and triplet (t) for one proton. The methine proton of the thiazepine nucleus resonates at around δ 2.96 as a triplet with coupling constants of nearly 12.8 Hz. This signal is observed as a triplet instead of a doublet of a doublet (dd) because two J-values accidentally are the same and two inner lines of the quartet occur at the same point, appearing as a single line of double the intensity22. The two methylene protons displayed two signals: a doublet of doublet at around δ 3.40 with coupling constants of nearly 13.8 Hz and 4.8 Hz and a doublet of doublet at around δ 5.16 with coupling constants of nearly 10 or 10.4 Hz and 5.2 Hz. Scheme 1 |
 |
BIOLOGICAL EVALUATION |
| In vivo anti-inflammatory activity |
| All the newly synthesized benzothiazepines (4a–g) were screened for their in vivo anti-inflammatory activity by paw edema method. Wister rats were used in the study were fed in house diet and water adlibitum and maintained at 12-12h dark light cycle, 25 C. Animals were administered Diclofenac 10 mg/kg, or test compound 10 mg/kg p.o., (n=3) two hours prior to injection of 0.1% formaldehyde in the paw. The anti-inflammatory was then calculated 120 minutes after induction and presented in Table–1 as the mean paw dimension in addition to the percentage inhibition. Paw dimension was measured by digital vernier calliper (Mitutoya, Japan.) |
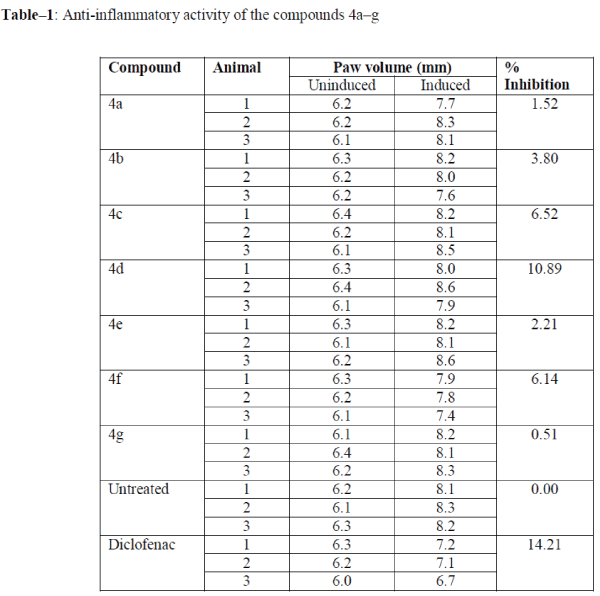 |
| Out of the seven compounds tested, three compounds (4c, 4d and 4g) showed significant anti-inflammatory activity. Among these compounds, the compound 4d (R3=-Cl) was found to be highly active with 10.89 % inhibition activity, while 4c and 4g with –Br and –H groups were also found have a respective inhibition rate of 6.52 % and 6.14 %. |
| However, the compounds 4a and 4g with methyl groups were found to be less active, with 1.52 and 0.51 % inhibition, respectively. The 4d with chloro group displayed considerable potent anti-inflammatory activity (10.89 % inhibition) comparable with diclofenac (14.21 % inhibition). However, none was found to be superior to the reference drug. |
CONCLUSION |
| In conclusion, the present investigation reports the synthesis of 1, 5 benzothiazepine derivatives and the evaluation of their anti-inflammatory activity (Figure 1). |
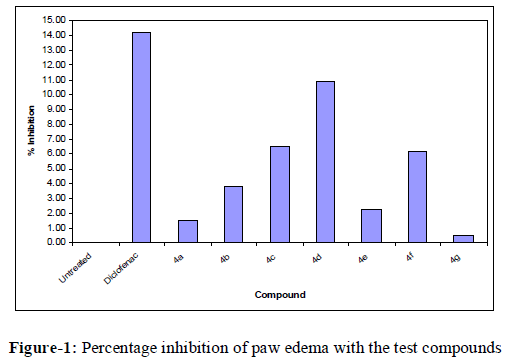 |
| The submission pattern of the 1, 5 benzothiazepine was rationalized to be correlated to the aryl heterocyclic template. Among all tested compounds, chloro-substituted benzothiazepine derivative 4d showed the highest antiinflammatory activity (10.89 % inhibition) that was comparable to diclofenac (14.21 % inhibition), while compounds 4c and 4g displayed good anti-inflammatory activity (6.52% and 6.14 % inhibition), respectively). However, none of the newly synthesized compounds were found to be superior to the reference drug. |
EXPERIMENTAL |
| All the recorded melting points (°C) were determined in the m.p. apparatus (Model: KI-11 [MP-D]), Make: Kumar Sales Corporation, Mumbai, India). IR spectra were recorded on a Perkin–Elmer FTIR spectrophotometer on a KBr disc. 1H NMR spectra were recorded on a Bruker ARX spectrometer with peak values shown in δ ppm using SiMe4 as the internal standard when measured in CDCl3 or DMSO-d6. Signal multiplicities are represented by s (singlet), d (doublet), t (triplet), m (multiplet), and q (quartet). Mass spectra were obtained by the Finnigan mass spectrometer. Substituted phenols and required chemicals for preparation of precursors were purchased from a commercial chemical company. TLC was performed on pre-coated silica gel glass plates (Kieselgel 60, 254, E. Merck, Germany) |
| General procedure for the preparation of chalcone (3a–g) |
| A 100 mL of conical flask was charged with an equivalent quantity of 1-(4-chlorophenyl)-1H-pyrazole-4- carbaldehyde 1 (0.01 mol) and with o-hydroxyacetophenones 2a–e (0.01 mol) in EtOH as a solvent. A solution of 40% KOH (5 mL) solution was added and the resulting mixture allowed remaining for 24 hours at room temperature. The progress of the reaction was monitored by TLC. After completion, the resulting mixture was poured into ice water and then neutralized with ACOH. The solid was obtained by filtration, dried and purified by recrystallization from ethanol, and column chromatography was applied to create the products 3a–g. |
| 3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-1-(2-hydroxy-5-methylphenyl)prop-2-en-1-one (3a) |
| Yield 76%; m.p. 164–166 °C; FT-IR (KBr) v/max (cm-1): 3431, 3020, 2412, 1646, 1584, 1215, 756; 1H NMR (400 MHz, CDCl3): 2.35 (3H, s, CH3), 6.91 (1H, d, J = 8.44 Hz), 7.30 (1H, d, J = 8.52 Hz), 7.44–8.16 (9H, m, ArH), 12.67 (1H, s, -OH); 13C NMR (100 MHz, DMSO-d6): 20.04, 117.60, 119.98, 120.11, 120.23, 120.76, 127.82, 129.61, 129.95, 131.10, 135.40, 137.13, 137.84, 141.7, 116.06, 193.33; MS, ES+1 mode (m/z): 339.08 (M+1). |
| 3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-1-(5-fluoro-2-hydroxyphenyl)prop-2-en-1-one (3b) |
| Yield 69%; m.p. 200–202 °C; FT-IR (KBr) v/max (cm-1): 3130, 2468, 1638, 1573, 833, 793; 1H NMR (400 MHz, DMSO-D6): 7.10 (1H, d, J = 8.52 Hz), 7.50 (1H, d, J = 8.58 Hz), 7.81–-9.22 (9H, m, ArH), 12.79 (1H, s, -OH); MS, ES+1 mode (m/z): 343.06 (M+1). |
| 1-(5-bromo-2-hydroxyphenyl)-3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (3c) |
| Yield 72%; m.p. 176–178 °C; FT-IR (KBr) v/max (cm-1): 3439, 1676, 1556, 834; 1H NMR (400 MHz, DMSO-D6): 6.95 (1H, d, J = 8.4 Hz), 7.08 (1H, d, J =8.8 Hz), 7.56–8.52 (9H, m, ArH), 13.2 (1H, s, -OH); MS, ES+1 mode (m/z): 402.98 (M+1). |
| 1-(5-chloro-2-hydroxyphenyl)-3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (3d) |
| Yield 75%; m.p. 175–178 °C; FT-IR (KBr) v/max (cm-1): 3150, 2351, 1682, 1567, 822; 1H NMR (400 MHz, DMSO-D6): 6.99 (1H, d, J = 8.44 Hz), 7.32 (1H, d, J = 8.52 Hz), 7.54–9.2 (9H, m, ArH), 12.40 (1H, s, -OH); MS, ES+1 mode (m/z): 359.03 (M+1). |
| 1-(3, 5-dichloro-2-hydroxyphenyl)-3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (3e) |
| Yield 71%; m.p. 260–261 °C; FT-IR (KBr) v/max (cm-1): 3435, 1676, 1596, 772; 1H NMR (400 MHz, DMSO-D6): 7.00 (1H, d, J = 8.44 Hz), 7.34 (1H, d, J = 8.52 Hz), 7.52–9.02 (8H, m, ArH), 13.02 (1H, s, -OH); MS, ES+1 mode (m/z): 392.99 (M+1). |
| 3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-1-(2-hydroxyphenyl) prop-2-en-1-one (3f) |
| Yield 62%; m.p. 168–171 °C; FT-IR (KBr) v/max (cm-1): 3373, 2401, 1640, 1590, 772; 1H NMR (400 MHz, DMSO-D6): 7.01 (1H, d, J = 8.44 Hz), 7.30 (1H, d, J = 8.52 Hz), 7.61–9.12 (10H, m, ArH), 12.99 (1H, s, -OH); MS, ES+1 mode (m/z): 325.07 (M+1). |
| 1-(3-chloro-2-hydroxy-5-methylphenyl)-3-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)prop-2-en-1-one (3g) |
| Yield 74%; m.p. 258–260 °C; FT-IR (KBr) v/max (cm-1): 3338, 3001, 2352, 1678, 1573, 834; 1H NMR (400 MHz, DMSO-D6): 2.52 (3H, s, CH3), 6.86 (1H, d, J = 8.44 Hz), 7.01 (1H, d, J = 8.52 Hz), 7.60–9.13 (8H, m, ArH), 12.61 (1H, s, -OH); MS, ES+1 mode (m/z): 373.04 (M+1). |
| General procedure for the preparation of benzothiazepine derivatives (4a–g) |
| To a solution of chalcone (0.01 mol) (3a–e) in 10 mL of ethanol, 0.01 mol of o-amino thiophenol and 2–3 drops of glacial acetic acid were added. The reaction mixture was refluxed by heating for 6 hours, and the progress of the reaction was monitored by TLC. After completion of the reaction, the resulting solution was cooled and transferred into crushed ice. The solid product was filtered and recrystallized from EtOH to enable benzodiazepine derivatives 4a–e. |
| 2-(-2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]thiazepin-4-yl)-4-methylphenol (4a) |
| Yield 71%; m.p. 220–222 °C; FT-IR (KBr) v/max (cm-1): 3400, 3020, 1575, 1467, 771; 1H NMR (400 MHz, DMSO-d6)3,5: 2.24 (3H, s, CH3), 2.96 (1H, t, J=12.8 Hz thiazepine ring), 3.40 (1H, dd, J=12.8 Hz, 4.8 Hz thiazepine ring), 5.16 (1H, dd, J=10.0 Hz, 4.8 Hz thiazepine ring), 6.97–7.79 (13H, m, ArH), 14.24 (1H, s, -OH exchangeable); 13C NMR (100 MHz, DMSO-d6): 20.09, 35.57, 50.62, 117.48, 117.80, 119.72, 122.87, 125.06, 125.26, 126.35, 126.76, 127.38, 129.45, 129.63, 130.25, 130.43, 134.63, 135.28, 138.33, 139.59, 148.74, 159.46, 174.28; MS, ES+1 mode (m/z): 446.10 (M+1). |
| 2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]thiazepin-4-yl)-4-fluorophenol (4b) |
| Yield 67%; m.p. 168–171 °C; FT-IR (KBr) v/max (cm-1): 3400, 3420, 1416, 771; 1H NMR (400 MHz, DMSO-d6): 2.87 (1H, t, J=13 Hz thiazepine ring), 3.65 (1H, dd, J=13.2 Hz, 5.2 Hz thiazepine ring), 5.4 (1H, dd, J=12.3 Hz, 5.3 Hz thiazepine ring), 7.01–8.80 (13H, m, ArH), 13.98 (1H, s, -OH exchangeable); MS, ES+1 mode (m/z): 450.08 (M+1). |
| 4-bromo-2-(2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]thiazepin-4-yl)phenol (4c) |
| Yield 69%; m.p. 158–160 °C; FT-IR (KBr) v/max (cm-1): 3483, 1476, 1215, 668; 1H NMR (400 MHz, CDCl3): 2.97 (1H, t, J=11.48 Hz thiazepine ring), 3.39 (1H, d, J=12.92 Hz thiazepine ring), 5.19 (1H, d, J=5.16 Hz thiazepine ring), 7.09–7.80 (13H, m, ArH), 12.93 (1H, s, -OH exchangeable); MS, ES+1 mode (m/z): 510.00 (M+1). |
| 4-chloro-2-(2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4] Thiazepin-4-yl)phenol(4d) |
| Yield 72%; m.p. 165–168 °C; FT-IR (KBr) v/max (cm-1): 3387, 1476, 755, 669; 1H NMR (400 MHz, CDCl3): 2.80 (1H, t, J=12.8 Hz thiazepine ring), 3.76 (1H, dd, J=12.9 Hz, 5.0 Hz thiazepine ring), 5.6 (1H, dd, J=12.6 Hz, 5.2 Hz thiazepine ring), 6.91–8.82 (13H, m, ArH), 14.10 (1H, s, -OH exchangeable); MS, ES+1 mode (m/z): 466.00 (M+1). |
| 2,4-dichloro-6-(2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]thiazepin-4-yl)phenol (4e) |
| Yield 70%; m.p. 180–182 °C; FT-IR (KBr) v/max (cm-1): 3393, 1582, 775, 669; 1H NMR (400 MHz, CDCl3): 2.85 (1H, t, J=12.4 Hz thiazepine ring), 3.75 (1H, dd, J=13.08 Hz, 4.72 Hz thiazepine ring), 5.36 (1H, dd, J=11.72 Hz, 5.0 Hz thiazepine ring), 6.41–8.77 (12H, m, ArH), 15.79 (1H, s, -OH exchangeable); MS, ES+1 mode (m/z): 500.01 (M+1). |
| 2-(-2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]thiazepin-4-yl)phenol (4f) |
| Yield 61%; m.p. 141–143 °C; FT-IR (KBr) v/max (cm-1): 3393, 1599, 769, 669; 1H NMR (400 MHz, CDCl3): 2.83 (1H, t, J=12.2 Hz thiazepine ring), 3.65 (1H, dd, J=12.68 Hz, 4.62 Hz thiazepine ring), 4.99 (1H, dd, J=12.74 Hz, 4.8 Hz thiazepine ring), 6.40–9.00 (14H, m, ArH), 14.67 (1H, s, -OH exchangeable); MS, ES+1 mode (m/z): 432.09 (M+1). |
| 4-chloro-2-(2-(1-(4-chlorophenyl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4] thiazepin-4-yl)-6-methylphenol (4g) |
| Yield 73%; m.p. 186–188 °C; FT-IR (KBr) v/max (cm-1): 3444, 3002, 1455, 794, 681; 1H NMR (400 MHz, DMSO-d6): 2.09 (3H, s, CH3), 2.92 (1H, t, J=12.4 Hz thiazepine ring), 3.40 (1H, dd, J=13.2 Hz, 5.2 Hz thiazepine ring), 5.14 (1H, dd, J=10.4 Hz, 5.2 Hz thiazepine ring), 6.92–7.95 (12H, m, ArH), 14.33 (1H, s, -OH exchangeable); MS, ES+1 mode (m/z): 480.06 (M+1). |
| Pharmacological Assay |
| Male Wistar rats were used for this study. They were fed an in-house diet and water and kept at a 12–12 hour dark/light cycle, 25C. Food was withdrawn 12 hours before and during experimental hours. The animals were arbitrarily divided into sets, each consisting of three rats. One group of three rats was kept as a control group. Such rats were administered Diclofenac 10 mg/kg, or test compound 10 mg/kg p.o., (n=3) two hours prior to injection of 0.1% formaldehyde in the paw. Paw dimensions were measured by digital vernier callipers (Mitutoto, Japan). |
ACKNOWLEDGEMENTS |
| One of the authors (Sharad N. Shelke) is thanks to University Grant Commission for financial assistance. The authors would like to thank the director of the Deshpande Laboratory (Bhopal, India) for the tests of anti-inflammatory activity. We are also grateful to Dr. A. B. Nikumbh, Head, Dept. of Chemistry, S.S.G.M. College, Kopargaon, Ahmednagar (MH) for providing research facilities and constant encouragement. |
References |
|