e-ISSN: 2319-9849
e-ISSN: 2319-9849
Department of Chemistry, Maharaj Singh College, Saharanpur, Uttar Pradesh, India
Received date: 24/12/2015; Accepted date: 21/07/2016; Published date: 27/07/2016
Visit for more related articles at Research & Reviews: Journal of Chemistry
Indoles, Sulpha/substituted, Pharmacological activity, Anti-tuberculosis activity, Anti-inflammatory activity.
The organic chemist employing the art of synthesis, have been responsible for the development of vast majority of drug used in modern system of medicine. Many founding members of medicinal chemistry were interesting not only in natural but also on the effect of synthetic compounds on living system.
Heterocyclic compounds are abundant in nature, great significance to life because their structural subunits exist in many natural products such as vitamins, hormones, antibiotics etc. Nitrogen containing heterocyclic compounds play important role in medicinal chemistry and also contribute to the society by helping in different processes.
Simple nitrogen containing heterocycles attached to sulphonamido moieties have received a large amount of attention in literature, as a consequence of their exciting biological properties and their role as pharmacophores of considerable historical importance. Heterocyclic sulphonamides are used as carbonic anhydrase inhibitors [1-3], anti-bacterial agents [4], anti-cancer, anti-inflammatory and analgesic agents [5], β- 3-adrenergic receptor agonistics [6]. Although the chemistry of Indole [7] has been investigated for more than 100 years as summarised in Scheme 1, recent times have been development of new indole chemistry, such as lithiated Indoles and indole radicals for use in synthesis [8,9].
Indoles are one of the most important nitrogen containing heterocyclic compounds (Figure 1). The Indole nucleus is important moiety found in a large number of natural or synthetic alkaloids [8,10]. One of the naturally occurring indoles, tryptophan, has a high sensitivity of tryptophyl residue in proteins and oxidation of tryptophan has been implicated in the photo degradation and photo yellowing of wool [11]. Nakazawaet al. [12] have prepared several indole derivatives and tested for their anti-thrombotic and allergy inhibitor activity.
A large number of heterocyclic compounds containing indole ring are associated with diverse pharmacological importance such as analgesic [13], anti-allergic [14], anti-bacterial [15], anticonvulsant [16,17], antifungal [18], antihistaminic [19], anti-inflammatory [20-22], anti-cancer [23,24] etc.
Based on above mentioned research results, the aim of the present work was to synthesise a novel series of pharmacologically sulpha/substituted indoles by refluxing the mixture of phenacylbromide and sulpha/substituted phenylamine in presence of glacial acetic acid and cyclisation of resulting product. The synthesis of compounds was illustrated in Schemes 1, 2 and 3.
Materials
All the substituted phenylamine, α-haloacyl benzene and reference compound were purchased from Aldrich Chemical. Ethanol, Glacial acetic acid and all other reagents were purchased from S. D. Fine Chem. Analytical TLC was performed on precoated plastic sheet of silica gel G/UV-254 of 0.2 mm thickness (Macherey-Nagel, Germany).
General
The melting points of the newly synthesised compounds were determined by using melting point apparatus (MP-DSTID 2000V scientific) and were uncorrected. The IR spectra of the synthesised compounds were recorded on IR spectrophotometer (perkin Elmer 1605 series) using KBr pellets. 1HNMR spectra were recorded at 300 MHz on Bruker Ft. NMR spectrometer using CDCl3 and the chemical shift (δ) reported are in ppm, using TMS as internal reference.
Experimental method-scheme of the work
Synthesis of 2- phenyl sulpha/substituted indole: 4.3.3.1: Synthesis of 2-phenyl 4-methyl indole: Phenacyl bromide and 4- methyl aniline (1: 2) ratio was dissolved in 20 ml Ethanol. The reaction mixture was heated on water bath for 1 hour and then cooled. On cooling, a crystalline solid mass was seprated out, filtered and recrystallized from Ethanol. The crystalline solid mass was dissolved in 30 ml acetic acid and refluxed for 3 hours on water bath. On cooling, the yellow colour solid mass separated out, filtered washed and recrystallized from ethanol and pyridine/acetic acid (1:1).
Result: Yield=63%, Colour=DY, M.P.=167°C, Molecular formula=C15H14N (Found N=6.55%, Cal. N=6.73%)
Rf value=0.8324, IR (KBr) (ѵmax in cm-1)=3140 (-NH stretch for indole), 660 (-CH3), NMR (CDCl3) (δ in ppm): 2 4 [s, 3H, CH3], 6.85-7.50 [m, 5H. ArH], 7.45 [d, 4H, 3,5,6,7 H of indolering].
Synthesis of 2-Phenyl-4 [N1 -2-Pyrimidyl sulphonoamido benzene] indole: The procedure mentioned above (synthesis of 2-phenyl 4-methyl indole) was adopted for the synthesis of this compound.
Result: Yield=90%, Colour=Shining green yellow, M.P.=168°C, Molecular formula=C24H19N4O2S (Found N=12.87%, cal. N=13.88%) Rf value=0.9497. IR (KBr) (ѵmax in cm-1): 3250 (-NH stretch for indole) 1360 and 1140 (-SO2-vibration of -SO2NH2 group). NMR (CDCl3) (δ in ppm): 9.1 [s, 1H, SO2NH2], 7.8 [s, 3H,4,5, and 6H pf pyrimidyl group], 7.5[d, 4H, -C6H4-SO2NH2], 7.35 [d, 4H, 3, 5, 6 and 7H pf indole ring], –7.2 [m, 5H, C6H5 at position of indole ring].
Synthesis of 2-phenyl-4 [N1-2-(3,5 dimethyl) pyrimidylsulphonoamidobenzene] indole: The procedure mentioned above (synthesis of 2-phenyl 4-methyl indole) was adopted for the synthesis of this compound.
Result: Yield: 92%, Colour=Light Brown, M.P.=170°C, Molecular formula=C26H23N4O2S, (Found N=11.92%, Cal. N=12.30%), Rf value=0.9053, IR (KBr) (ѵmax in cm-1) : 3215 (-NH stretch for indole), 665 and 670 (-CH3 for pyrimidyl group), 1360 and 1145 (-SO2–Vibration of–SO2NH2 group). NMR (CDCl3) (δ in ppm): 9.3 [s, 1H, -SO2NH2], 7.9 [s, 4 and 6H of pyrimidyl group], 2.4 [s, 6H, CH3], 7.30 [d, 4H, 3, 5, 6 and 7H of indole ring], 7.1-7.2 [m, 5H, C6H5 at position of indole ring].
Synthesis of 2-phenyl-4-(N1 -2 pyridylsulphonoamidobenzene) indole: The procedure mentioned above (synthesis of 2-phenyl 4-methyl indole) was adopted for the synthesis of this compound.
Result: Yield = 67%, Colour=Colourless flakes, M.P.= 156°C, Molecular formula= C25H20O2N3S, (Found N=9.57%, Cal. N.= 9.85%), Rf value=0.7832, IR (KBr) (ѵmax in cm-1): 3250 (-NH stretch for indole) 1355 and 1130 (-SO2–Vibration of –SO2NH2 group), NMR (CDCl3) (δ in ppm): 9.3 [s, 1H,-SO2NH2], 7.8 [s,3H,4 and 5H of pyridyl group], 7.3 [d, 4H, -C6H4–SO2NH2], 7.35 [d, 4H, 3,5,6 and 7H of indole ring], 7.12 (dd, N,-C5H4N) 7.1 – 7.2 [m, 5H, C6H5 at position of indole ring].
Synthesise of 2-phenyl-3 (sulphonoamidobenzene) azo 4-methyl indole: 4.3.2.1 Synthesis of 2-sulphonoamidobenzene azo substituted phenyl amino N-Phenacyl amine: 2- Sulphonoamidobenzene (5 gm) was dissolved in dil. HCl (4 ml), water in sufficient amount and cooled to 0-5°C. Aqueous solution of sodium nitrite (4 gm) gradually added to sulphonoamidobenzenehydrochloride. The diazonium salt solution so obtained was filtered into a well cooled stirred mixture of sodium acetate (10 gm) an N-phenacyl 4-methyl phenyl amine in ethanol (20 ml) and shaken vigorously. A coloured precipitated, separated out, filtered dried and recrystallized from ethanol giving shining pale yellow needles.
Result: Yield=72%, M.P.=183°C, Molecular formula=C32H22N4Cl2 (Founded N=10.21%, Cal. N=10.52%), Rf Value=0.4232, IR (KBr)=1590 cm-1 (N=N), 3140 cm-1 (N-H Scratching of sulphonoamido group), 1350 cm-1 (SO2 Vibration of sulphonoamide group).
Synthesis of 2-phenyl-3-(2-sulphonoamidobenzene) azo-4-methyl indole: 2 sulphonoamidobenzeneazo 4-methyl phenyl N-phenacyl amine (3 gm) was dissolved in sufficient amount of glacial acetic acid and refluxed on water bath for four hours. On cooling, a coloured crystalline solid compound separated out, filtered, recrystallized from ethanol.
Result: Colour: SOF, Yield=78%, M.P. = 172°C, M.F.=C26H21N4O2ClS (Found N=11.13%, Calculated N=11.47%), IR (KBr)=3260 cm-1 (N-H stretching of sulphonamide and indole), 1580 cm-1 (N-H bending), 1440 cm-1 (N=N Stretching), 1370 cm-1 and 1140 cm-1 (-SO2-Vibration of Sulphonamide), NMR (CDCl3) (in ppm) : 8.4 (s, 1H,-SO2NH2), 8.05 (s, 1H, indolyl NH), 7.5 (d, 4H,- N-C6H4- SO2NH2), 7.4 (d, 4H,-3, 5, 6, 7 -H of indole), 7.1 -7.2 (m, 5H, aromatic protons).
By adopting above procedure 4-chloro, 4-Fluoro, 4-Hydroxyl, 4-Nitro, 2-sulphonoamido-benzene, N1-2- pyrimidyl. Sulphonoamidobenzene, N1-2 (3, 5 dimethyl pyramidyl sulphonoamidobenzene, 2, 3 dimethyl 1- phenyl pyrazolone, N1-2 guanyl sulphonomido- benzene, N1-2 pyridylsulphonoamidobenzene, N1-2 thiazolyl sulphonomidobenzene, N1-2 acetyl sulphonomidobenzene. N1-2 Quinoxalyl sulphonoamidobenzene and N1-2 thiazolyl sulphonoamidobenzene derivatives were synthesised and the newly synthesized compound is recorded in Table 1.
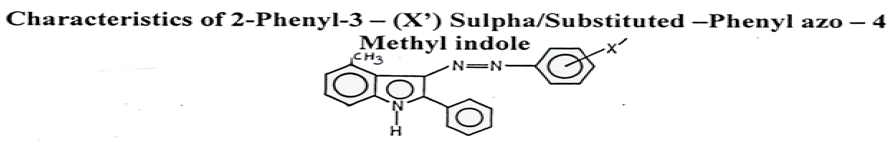
| S. No | Substituted Group X’ | M.P.°C | Yield % | Colour | Molecular Formula | Nitrogen Found | Rf value |
|---|---|---|---|---|---|---|---|
| 1. | 4-Chloro | 112°C | 75% | SP | C21H18N3Cl | 11.82%12.10% | 0.7932 |
| 2. | 4-Fluoro | 117°C | 60% | DY | C21H18N3F | 12.56%12.72% | 0.8651 |
| 3. | 4- Nitro | 128°C | 72% | SYN | C C21H18N4O2 | 15.31%15.64% | 0.8266 |
| 4. | 4-Hydroxy | 135°C | 66% | DYB | C21H19N3O | 12.49%12.76% | 0.8533 |
| 5. | 2-Sulphono- amidobenzene | 148°C | 64% | SOF | C27H24N6O2S | 11.62%11.96% | 0.8754 |
| 6. | N’-2Pyrimidyl sulphonoamidobenzene | 147°C | 75% | SP | C31H26N6O2S | 15.05%15.38% | 0.8652 |
| 7. | N’-2(3,5 dimethyl) Pyrimidylsulphonoamidobenzene | 155°C | 82% | GY | C33H30N6O2S | 14.21%14.63% | 0.7531 |
| 8. | 2,3-Dimethyl-1 phenyl Pyrazolone | 152°C | 86% | SYN | C38H34N5 | 12.22%12.50% | 0.7745 |
| 9. | N’-2Guanyl Sulphonoamidobenzene | 168°C | 81% | DYB | C28H26N6O2S | 16.17%16.47% | 0.7654 |
| 10. | N’-2Pyridyl Sulphonoamidobenzene | 165°C | 85% | DY | C32H27N5O2S | 12.53%12.84% | 0.7322 |
| 11. | N’-2 ThiazolylSulphonoamidobenzene | 172°C | 90% | SP | C30H25N5O2S2 | 12.33%12.70% | 0.8351 |
| 12. | N’-2 acetyl Sulphono -amidobenzene | 165°C | 65% | DY | C29H26N4O3S | 10.61%10.98% | 0.8351 |
| 13. | N’-2 Quinoxalyl Sulphonoamido benzene | 144°C | 73% | DY | C27H2864O2S | 16.57%16.80% | 0.8752 |
DY=Dull yellow, SPY=Shining Pale Yellow, GY=Golden Yellow, LY=Light Yellow, BY=Bright Yellow, RN=Red Needles, BON=Bright Orange Needles, OY=Orange Yellow, PY=Pale Yellow.
Table 1: Characteristics of 2-phenyl-3-(X’) Sulpha/Substituted-Phenyl azo-4 Methyl indole.
Prepration of 2-phenyl -5-sulpha/substituted- 3-phenyl substituted azo indoles: Sulpha/Substituted phenyl amine was dissolved in HCl, water is added in sufficient amount and cooled to 0°C. Aqueous solution of sodium nitrite was gradually added to sulpha/substituted phenyl amine hydrochloride. The diazonium salt solution so obtained was filtered into a well cooled stirred mixture of sodium acetate and sulpha/substituted phenyl amino N-phenacyl phenyl amine in ethanol and shaken vigorously, precipitate separated out, filtered, dried and recrystallizes from ethanol giving shining coloured needles of sulpha/ substituted phenyl azo substituted phenyl amino N- phenacyl phenyl amine. Sulpha/substituted phenyl azo phenyl amino N- phenacyl phenyl amine was dissolved in glacial acetic acid and refluxed on water bath for half an hour. On cooling a crystalline solid compound separated out and recrystallized from ethanol
Synthesis of 2- phenyl-5-sulphonoanifo benzene-3-phenyl fluoroazo indole: A light yellow crystalline yellow powder, M.P.=172°C, yield=65%, molecular formula=C26H19FN4SO2, Analytical calculated=(C=66.37%, H=4.07%, F=4.02%, N=11.87%, S=6.78%, O=6.78%), Found (C=66.32%, H=4.04%, F=4.02%, N=11.57%, S=6.73% O=6.73%). UV (λmax) = 280, IR (KBr) vmax in cm-1 1325 (C-F), 760 (C-C), 1245 (C-N), 1560 (C=C or aromatic ring), 3040 (aromatic C-H), 3345 (N-H), 1445 (N=N), 1153 (SO2), 3280 (NH2), 1NMR (CDCl3) δ in ppm: 5.9 (b, IH, NH), 7.75-6.40 (m, 16H, Ar-H), 11.5 (b, 2H, SO2NH2).
Synthesis of 2-phenyl-5- benzene sulphonamide- 3-phenyl chloroazoindole: A light yellow crystalline powder, M.P.=170- 172°C, Yield=69%, molecular formula=C26H19ClN4SO2, (486.98), Anal Cal. C=64.13%, H=3.93%; Cl=7.28%, N=11.50%; S=6.58%; O=6.57%, Found: C=64.11%: H=3.90%: Cl=7.25%; N=11.49%; S=6.55%; O=6.55%. UV (λmax)=277. IR (KBr) vmax cm-1 670 (C4-Cl), 760 (C–C), 1240 (C-N), 1565 (C=C or aromatic ring), 3045 (aromatic C-H), 3340 (N-H), 1445 (N=N), 1150 (SO2), 3280 (NH2), 1NMR (CDCl3) δ in ppm: 5.9 (b. IH, NH), 7.65-6.75 (m. 16H, Ar-H), 11.5 (b, 2H, SO2NH2).
Anti-tuberculosis activity
All the newly synthesised compound were tested for their anti-tuberculosis activity against M. tuberculosis H37 Rv by bactec 460 radiometric system at Southern Research Institute, Frederick Research Centre, Frederick, MD.
Primary Screening of invitro tuberculosis activity was conducted at concentration of 12.5 μg/ml against Mycobacterium tuberculosis H37 Rv in BACTEC 12B medium using BACTEC 460 radiometric system. The anti-tuberculosis activity of all newly synthesised compounds are compared with the standard Rifampin (which has 96% inhibition at MIC of 0.31 μg/ml. Some of newly synthesised compounds were screened for their anti-tuberculosis activity. Some of them showed significant activity recorded in Table 2.
| S. No. | Name of Compound | M. T.* |
|---|---|---|
| 1 | 2-phenyl 4-Hydroxy Indole | (+) |
| 2 | 2 phenyl -4 – (N1-2thiazolyl sulphonoamidobenzene)Indole | (+) |
| 3 | 2-phenyl–4–methyl Indole | 0 |
| 4 | 2–phenyl-4–Fluoroindole | 0 |
| 5 | 2- phenyl–4–(N1 -2-Guanylsulphonoamido (+) benzene) indole | (+) |
*M.T.=M. Tuberculosis H37Rv, (+)=Positive
Table 2: Anti-tuberculosis activity data of newly synthesized compounds.
Anti-inflammatory activity
All newly synthesised compounds were screened for their anti-inflammatory activity with the help of following method as compared to standard Indomethacin at 200 μg/ml. Mice of either sex weighing between 15 and 25 gm were divided into groups of 5 each. Carrageenin solution (1%, 0.025 ml) in normal saline was injected in the left planter aponeurosis, after one hour of the oral feeding of drug. One group acted as control and received only the vehicle and another group received a standard antiinflammatory compound (Indomethacin). Both the hind limbs of all the groups were cut 4 hours after the carrageenin injection at level of the ankle Joint. Difference between the weight of the left and right limbs gave the amount of edema developed. Difference in the amount of edema developed in each group from the control group in used to calculate the percentage inhabitation. Some of newly synthesised compound show significant anti-inflammatory activity recorded in Table 3.
| S.No. | Name of Compound | Approximate ALD50Dose (mg/kg) Mice P.O. | Anti-inflammatory activity of inhibition | |
|---|---|---|---|---|
| 1- | 2-phenyl-4(2,3 di methyl -1phenyl pyrazoloneindole | >1000 | 200 | 74 |
| 2- | 2- phenyl -4 (N1-2phenyl sulphonamidophenylIndole | 681 | 200 | 79 |
| 3- | 2- phenyl-4 (N1-2acetyl sulphonamido benzene) Indole | 825 | 168 | 77 |
| 4- | 2 phenyl-4-nitro Indole | 681 | 198 | 79 |
| 5- | 2-phenyl-4-Fluoro Indole | 825 | 173 | 62 |
Table 3: ALD50 and Anti-inflammatory activity data.
The author wish to express his sincere thanks to Principal, Maharaj Singh College, Saharanpur for providing necessary facilities and also wish to express their sincere thanks to Director, CDRI Lucknow and Southern Research Centre Frederick, Frederick Research Centre, Frederick (M.D.) for helping in spectral studies and in biological activity of synthesised compounds.