e-ISSN: 2319-9849
e-ISSN: 2319-9849
1 Faculty of Pharmacy, University of Zawia, Al-Zawia, Libya
2Raghavendra Institute of Pharmaceutical Education and Research, Ananthapur, India
Received: 01/02/2013; Revised: 19/03/2013; Accepted: 26/03/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
A molecular manipulation efforts were made at β carbon of α, β unsaturated site of nalidixic acid by Michael addition, in expectation to get newer chemimanipulated derivatives with potent/modified antimicrobial spectrum. At the ends of chemimanipulative work, totally 5 derivatives were synthesized and characterized by spectral data. All the derivatives were screened for its antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aureginosa, and Proteus vulgaris at a concentration of 100μg/disc with the same dose of nalidixic acid as control by agar plate disc diffusion method. The results revealed that hydrazine substituted tricyclic derivatives exhibited potent antibacterial activity gram +ve rather than against gram –ve, however, potentiation were found to be against gram +ve and gram –ve bacteria.
Nalidixic acid; derivatives; chemimanipulation; antimicrobial agents.
The molecular manipulation of structure in drug design is to get a highly active compound with minimal side effect still draws continued interest in the field of organic and medicinal chemistry. Combination of two or more active moiety into one moiety or the elimination, introduction or substitutions of certain groups in a drug molecule is a common procedure of manipulation and this can probably result in its increase of biological activity and potency of molecules and particularly it is done on chemotherapeutic agent to prevent the development of resistance by infectious organisms.
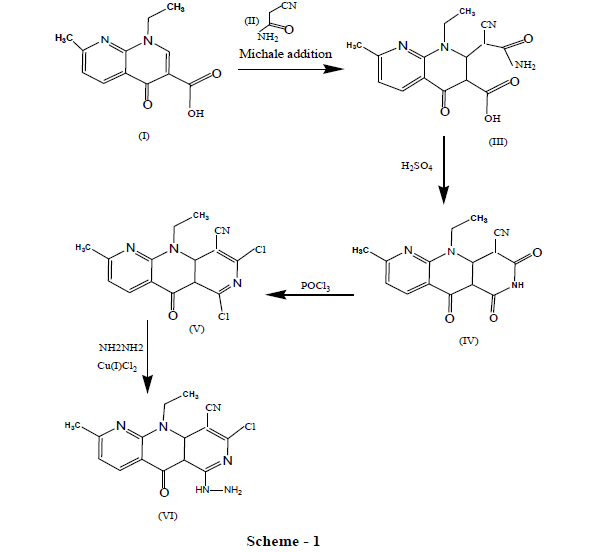
Nalidixic acid is the first 4 - quinolone antibacterial agent reserved for urinary tract infection, having α, β unsaturated carboxylic acid at 2, 3 positions of 1,8 naphthyridin- 4 - moiety [1,2]. The antibacterial spectrum of nalidixic acid known to exhibit resistance towards most of the common pathogens like staphylococcus, Bacillus, psuedomonas and proteus species which stands for urinary tract infection [3]. So in continuation of our earlier work [4] it was directed to do modification at β carbon of α, β unsaturated carboxylic acid of nalidixic acid by Michaels addition [5] to introduce possible moiety and followed by cyclization to afford newer tricyclic derivatives in view to obtain molecular manipulated derivatives with potent/modified antimicrobial spectrum. The parent compound nalidixic acid (I) was treated with cyano acetamide (II) in presence of pyridine/NiCl2 (Michael addition) [6] afforded 2,3 dihydro 2- substituted nalidixic acid (III) in excellent yield as intermediate. Further the intermediate was subjected to thermal cyclization in presence of conc. H2SO4, gave tricyclic derivative (IV) followed by treatment with Phosphorus oxy chloride yielded dichloro derivatives of cyano pyridino naphthrydin -4 – ones (V). The compound (V) was treated with various hydrazine hydrate reactants resulted compound (VI). The purity and authentication of the compound was tested on silica gel G coated TLC plate and the structures were assigned based on the IR and PMR data.
TLC was seen on Silica gel G plates using benzene-chloroform (9: 1) as irrigant. Open capillary tube using cintex apparatus was used to determine the melting point of the compounds and it was found to be decomposed in higher temperature. IR spectra were recorded (in Cm-1) using KBr pellet technique and H1 NMR was recorded (Chemical shift δppm) using TMS as internal standard.
Synthesis of 2,3 dihydro 2- substituted nalidixic acid (III); General procedure: 0.01 mole of Nalidixic acid (I) and cyano acetamide (II) 0.01 mole, NiCl2.6H2O (2.3g, 0.1 mole) were refluxed in mixture of 3:1 ratio of pyridine and ethanol for 3 hours (Michael addition).
Cooled mixture was acidified with concentrated HCl and the obtained precipitate was dissolved in sodium bicarbonate and reprecipitated with concentrated HCl. The product was dried and recrystallized with ethanol to afford compound (III) and single spot on TLC plate confirmed the purity of the compounds.
III: m.p. 201 °C, yield 65%, IR: 3423(OH and NH coupled, str), 3011, 3008 (Ar-H str), 2978(C-H str), 2344 (CN) 1712, 1682 (C=O str), 1620, 1585, 1508(C=C and C=N str), 1075(C-N str) and 756 (Ar-H def.out of plane).
PMR: 3.43 (s, 3H, CH3), 2.2 (t, 3H, CH3), 2.9 (q, 2H, -CH2-), 5.1-5.5 (dd, 3H -CH-CH-CH of naphthyridine, complex), 7.2-7.93 (m, 4H, Ar-H and -NH-), 10.3 (s, 1H, COOH)
Synthesis of IV: A mixture of compounds III (0.01 mole) and 5 ml of sulphuric acid were refluxed for 2 hours at 110oC. The reaction mixture was cooled and poured into the crushed ice. The obtained product was filtered and washed until the filtrate was neutral to PH paper. The product was dried and recrystallized with ethanol to afford compounds IVand Single spot on TLC plate confirmed the purity of the compound.
IV: m.p. 348 °C, yield 55 %, IR: 3423(OH and NH coupled, str), 3012, 3008 (Ar-H str), 2978(C-H str), 2346 (CN) 1714, 1681 (C=O str), 1620, 1584, 1508(C=C and C=N str), 1077(C-N str) and 754 (Ar-H def.out of plane).
PMR: 3.42 (s, 3H, CH3), 2.21(t, 3H, CH3), 2.9 (q, 2H, -CH2-), 5.2-5.6 (dd, 3H -CH-CH-CHof naphthyridine, complex), 7.2-7.93 (m, 3H, Ar-H and -NH-)
Synthesis of compound V: To the 0.01 mole of compound IV, 10 ml of phosphorous oxy chloride was added and refluxed for 2 hrs. The resultant reaction mixture was poured on to crushed ice containing ammonium hydroxide. The resultant precipitate was washed with water and recrystallized from DMF. The compound structure was supported by the absence of absortion band at 1681Cm-1(Yield: 70%; m.p.187°C).
Synthesis of compound VI: General procedure: Compound V (0.01mole) and appropriate primary amino reactants (0.01 mole) in 30 ml of ethanol and catalytic amount of copper (I) chloride 0.005 mole triethanolamine heated under reflux for 5 hrs. The resultant reaction mixture was poured on to crushed ice. The resultant crude solid was separated and recrystallized from DMF to give VIa-d.
VI: m.p. 233 °C, yield 59 %,
IR: 3326(OH and NH coupled, str), 3010, 3006 (Ar-H str), 2979(C-H str), 2339(CN) 1719, (C=O str), 1614, 1581, 1508(C=C and C=N str), 1079(C-N str), 992 (C-Cl) and 761 (Ar-H def.out of plane).
PMR: 3.43 (s, 3H, CH3), 2.2 (t, 3H, CH3), 2.9 (q, 2H, -CH2-), 5.3-5.7 (dd, 2H -CH-CH of naphthyridine), 7.4-8.04 (m, 8H, Ar-H and - NH-), 6.45 (s, 1H, NH), 6.92 (s, 2H, NH2).
Similarly others compounds were synthesized as shown in Scheme 1 and are listed in Table 1 with their physical properties.
Antimicrobial activity
A total of 5 new nalidixic acid derivatives were subjected to antibacterial screening against S.aureus, B.subtilis, P.aeruginosa, and P.vulgaris at a concentration of 100 μg/disc using the same dose of nalidixic acid as control and norfloxacin as a standard by agar plate disc diffusion method [7,8]. The solvent used was DMSO. The zone of inhibition was measured in mm and reported in Table 2.
All the synthesized compounds were exhibited activity against gram-positive organism with remarkable zone of inhibition. The compounds VI, exhibited wide broad spectrum activity indicated that Hydrazine substitution potentiate the molecule towards gram +ve rather than gram
-ve organisms. However the potentiation found to be superior for both gram +ve and gram –ve bacteria. Further Substitution of chlorine at ortho position of phenyl amino group enhances has not shown any remarkable activity.
Authors are thankful to Indian Institute of Technology, Chennai and IICT, Hyderabad, India for their help in spectral studies and to the management of Raghavendra Institute of Pharmaceutical Education and Research, Ananthapur, India for the provided facilities.