ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
P.Sujitha1, B.Subba Rao2, K.V.R.Murthy3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
This paper reports the photoluminescence properties of Ca2CeO4 phosphor with different concentrations of trivalent Europium as dopant. The phosphor is synthesized using the solid state diffusion reaction. Photoluminescence and thermoluminescence studies are done on the prepared phosphor. For crystal structie and surface morphology XRD and SEM micrographs of Ca2CeO4 : Eu phosphor was also studied. From XRD and SEM confirms the formation of nano phosphor in the range around 70nm.
Keywords |
| Photoluminescence (PL) , X-ray diffraction (XRD)Thermoluminescence (TL) ,solid state reaction (SSR). |
INTRODUCTION |
| The luminescence associated with Eu3+ contained in different host lattices has found applications in display devices due to its red light emission [1-3]. Oxide based luminescent materials exhibits good photoluminescence properties which are used as the display device phosphors. The rare earth materials exhibit sharp emission luminescence properties. The characterization of the prepared materials was done using PL,TL,XRD,SEM and particle size analysis techniques. |
EXPERIMENTAL |
| The phosphor sample was prepared by solid state diffusion reaction method. The phosphor Ca2CeO4 is prepared from the compounds calcium carbonate (CaCO3) and cerium oxide (CeO2). The prepared Ca2CeO4 phosphor is weighed and grounded into a fine powder using agate mortar and pestle about an hour. The grounded mixture was placed in an alumina crucible and heated from room temperature to 12000c in a muffle furnace with a heating rate of 50C/min. After reaching 12000c the phosphor heated for 3hours and the furnace was allowed to cool to room temperature along with the samples. |
| The basic reaction is as follows: |
| 2CaCO3 + CeO2 → Ca2CeO4 +2CO2 |
CHARACTERIZATIONS |
| The characterizations of prepared phosphors are done using Photoluminescence (PL), Thermoluminescence (TL), XRD, SEM and particle size analysis. The photoluminescence spectra were recorded at room temperature using spectroflurophotometermeter (SHIMADZU, RF5301PC) xenon lamp as excitation source. The thermoluminescence glow curves were recorded by TL glow curve reader and the irritation was done with Sr-90 beta source[3]. Using XRD technique the phase and crystallite size is found. The surface morphology and the particle sizes of the phosphors are observed from SEM. |
RESULTS AND DISCUSSIONS |
| (A)Photoluminescence study: |
| Fig I(a) and fig I(b) shows the PL spectra of Ca2CeO4 : Eu3+ when excited 254nm. The PL emission spectra of samples were recorded for the excitation wavelength of 254nm for different concentrations of Eu. The excitation of the Ca2CeO4 : Eu3+ phosphor with 254nm wavelength generates photoluminescence emission at 593,611 and 633nm with intensities of around 25a.u. The peaks at 611 and 633nm are due to electric dipole transition |
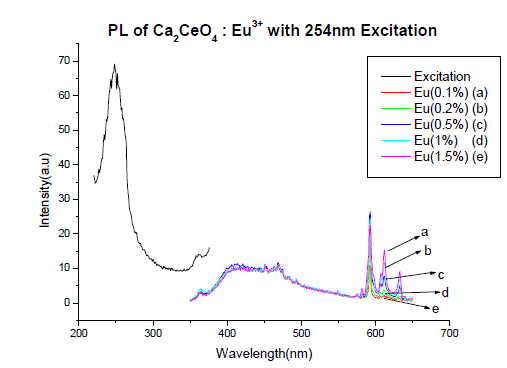 |
| Fig. 1 (A&B) are the Eu3+ emissions of Ca2CeO4 : Eu when exciting with 254nm which are 593, 611 and 633nm. From Fig. 1 (A&B) it is observed that there is a uniform PL emission intensity from 400 to 500nm followed by Eu3+ emissions. For better understanding the concentration of Eu and emissions of the phosphors are tabulated in table -1. |
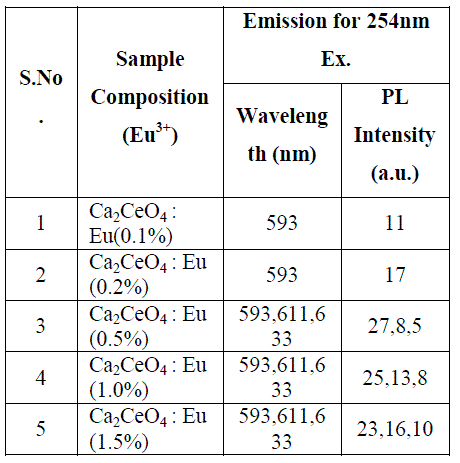 |
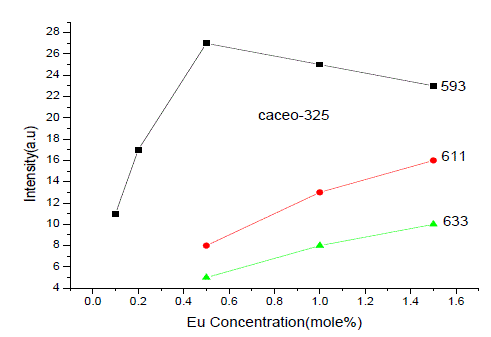
| From Fig. 1-B it is found that the intensity of 593nm increases linearly upto 0.5% of Eu concentration and then reduces its intensity without changing the emission wavelength. For 613nm peak, the intensity increases linearly, the same pattern is followed for 633nm peak. The peaks at 593, 611 and 633nm are the allowed transitions of Eu3+ emissions. The 593nm emitted peak is due to 5D0 → 7F1 transition of Eu and due to the magnetic dipole with energy 2.2ev. The peak at 611nm is due to 5D2 → 7F2 transition of Eu with energy 2.02ev and due to electric dipole of phosphor. The peak at 633nm is the allowed transition of Eu3+ which is due to 5D0 → 7F3 having an energy 1.98ev and also due to electric dipole. It is a thumb rule in the Eu doped phosphors; the emissions below to 600nm are due to magnetic dipole and above 600nm are due to electric dipole of phosphor whatever the excited wavelength may be[4-7]. Fig.2 is the Eu concentration in Ca2CeO4 versus intensity of 593, 611 and 633nm peaks when excited with 254nm. From fig.2 it is found the intensity of 593 nm peak intensity peak increases up to Eu concentration 0.5% and other two peaks intensity increase linearly till the Eu concentration in Ca2CeO4 is 1.5%. |
| (B).Thermoluminescence study: |
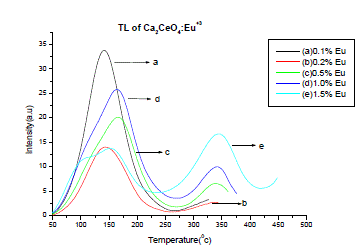
| Fig. 3 is the TL emission of Ca2CeO4 : Eu (5Gy beta dose). The TL of the phosphor is recorded immediately after beta irradiation. The TL is observed for different concentrations of Eu. When the Eu concentration is 0.1% in Ca2CeO4 a broad isolated and well resolved TL peak is observed at temperature 1420c with an intensity of 34a.u As the Eu concentration increases in Ca2CeO4 a small hump is observed at temperature of 3430C with an intensity of 17a.u when Eu is 1.5%. Table-2 is the intensities vs temperatures of various observed TL peaks in Ca2CeO4 :Eu |
 |
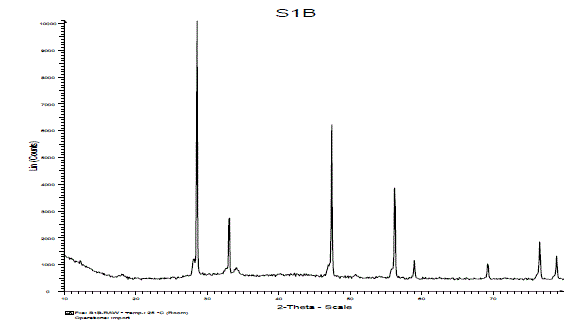
| (C).XRD Study: |
| The crystal structure of the product was examined by XRD analysis. Fig. 4 shows the X-ray diffraction pattern of synthesized sample Ca2CeO4 : Eu3+. From the XRD peaks in the diffraction pattern seems the phosphor may be formed single phase. The crystallite size of the particles of powder sample were calculated by Scherer’s formula i.e D=0.9lambda/βcos(theta). The average crystallite size of Eu doped Ca2CeO4 phosphor is 63nm.Which conclude us the solid state reaction method is good to prepare nano phosphors also. |
 |
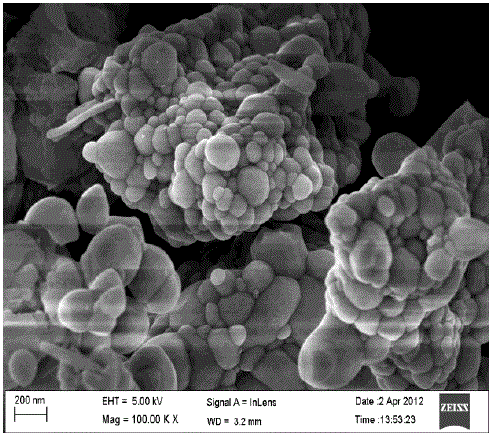
| (D).SEM Study: |
| Fig.5 shows the scanning electron micrograph of Ca2CeO4 : Eu3+. From the Scanning Electron Micrograph of Ca2CeO4 : Eu it is found the particles are distorted spheres in shape with various sizes from 50-300nm and also clusters are found. From the SEM micrograph the calculated average basal diameter of 77nm[8]. |
 |
| (E).Particle size analysis: |
| The particle size distribution histograms of Ca2CeO4 : Eu3+(1.5%) particle is shown in fig.6. From particle size histogram it is found that two maximum particle size of 4 microns and 12 microns are found. This allows us to draw a conclusion that two different sizes of phosphor may be existing and majority may be in a single phase. This variation of particle sizes may be due to agglomeration of nano particles to micrometer particles due to long storage of the phosphor before the Particle size analysis studies[8-11]. |
 |
CONCLUSIONS |
| Eu doped with Ca2CeO4 nano phosphor was synthesized via solid state diffusion technique. From the XRD study it is concluded the majority phase of the phosphor is in single phase. As the Eu concentration increases the dopant emission intensity increases. Which conclude us the solid state reaction method is good to prepare nano phosphors for display device applications due to the spherical shape of the phosphor particles. |
References |
|