ISSN ONLINE(2278-8875) PRINT (2320-3765)
ISSN ONLINE(2278-8875) PRINT (2320-3765)
Baby Pooja1, Satnam Singh Dub2, Parveen Lehana3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering
Currently, fossil fuels as main energy source to fulfil world’s energy demand is depleting fast. Their waste products are also causing global environment problems. One of the solutions is to replace the existing fossil fuel energy system by the non- fossil fuel energy system. The relative costs and CO2 emission reduction benefits of advanced non fossil fuel electricity generation. Utilization of photovoltaic (PV) generated electricity in power system detached from public grid usually requires both short term storage for compensation of day/night cycles as well as long term storage for smoothing the seasonal energy harvest distinction. This paper presents a model of small electricity energy generation based on solar- hydrogen fuel which resolves the long term storage problems of energy. In a solarhydrogen energy system, some photovoltaic array would provide current electricity demand while others would be used to produce hydrogen for storage and later use in fuel cells to generate electricity. The paper also presents the design of the system that is used between solar -hydrogen fuel system to generate non-stop electricity i.e., independent on weather climate or sun.
Keywords |
| Electrolysis process, load, multi-meters, PEM fuel cell, solar panel |
INTRODUCTION |
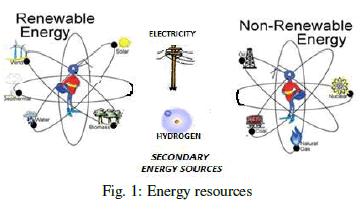
| Energy is a vital input for social and economic development. As a result of the generalization of agricultural, industrial, and domestic activities the demand for energy has increased remarkably, especially in emergent countries. This has meant rapid growth in the level of greenhouse gas emissions and the increase in fuel prices, which are the main driving forces behind efforts to utilize renewable energy sources more effectively, i.e. energy which comes from natural resources and is also naturally replenished. Despite the obvious advantages of the renewable energy, it presents important drawbacks, such as the discontinuity of generation, as most renewable energy resources depends on the climate, which is why their use requires complex design, planning, and control optimization methods. It is prudent that neither a stand-alone solar energy system nor a hydrogen energy system can provide a continuous power supply due to seasonal and periodical variations for stand-alone systems [1]. Different types of energy resources are shown in Figure. 1. All the basic necessities of life depend upon the sufficient and un-interrupted supply of energy. Yet we take it granted- and energy demand continues to grow year after year. This paper presents a model of small electricity energy generation based on solar-hydrogen fuel which resolves the long term storage problems of energy. In a solar-hydrogen energy system, some photovoltaic array would provide current electricity demand while others would be used to produce hydrogen electrolytically for storage and later use in fuel cells to generate electricity. |
 |
| In the long term, a hydrogen based economy will have an impact on all the sectors. Hydrogen provides what in many respects is an ideal energy carrier or energy currency. Life cycle assessment is a systematic analytic method that helps identify and evaluate the environment impacts of competing processes. |
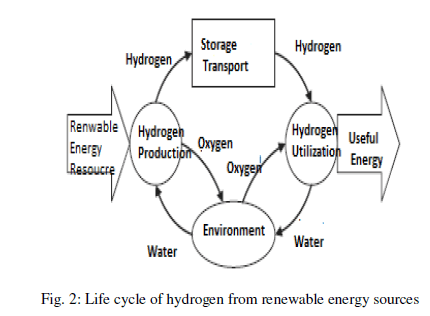 |
| In the way as shown in figure 2, a life cycle inventory is created which accounts for the total inputs and outputs of all flows attributable to the production of hydrogen [2]. Electricity produced from renewable energy sources could be used to make hydrogen and oxygen by electrolysis of water, and hydrogen recombined with oxygen in a fuel cell to generate electricity when needed as shown in Figure 3.This work addresses the issues relating to the development of a new concept for on board-hydrogen production for energy recovery [3]. |
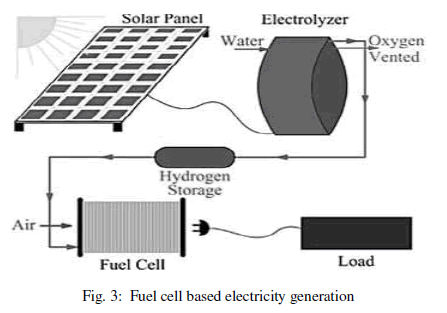 |
| Solar based hydrogen generation may be difficult during cloudy weather. Hence for uninterrupted energy supply, solar based systems should be supported by alternative mechanism of energy production. For example, solar-hydrogen storage based systems may help towards this objective as shown in figure 3. It allows testing of an energy management strategy to best satisfy the power demand of the fuel cell. No other energy generating technology holds the combination of benefits that fuel cells offer. Some of the benefits are as follows. |
| Energy security: Abundant source |
| Supply security: Efficient, modular, and fuel flexible |
| Physical security: Resources evenly distributed in nature |
| High reliability |
| High quality power and high efficiency – as high as 85% |
| Environmental friendly |
| The objective of this paper is to investigate temporal power analysis of Solar-hydrogen based fuel cell system. Section II explains Solar-hydrogen based fuel Cell System which consists of three parts i.e. solar panel, electrolysis process of water, and hydrogen fuel. The methodology is given in section III, results and discussions in section IV along with conclusions in section V. |
SOLAR-HYDROGEN BASED FUEL CELL SYSTEM |
| Solar-hydrogen based fuel cell system is a system which consists of three parts i.e. solar panel, electrolysis process of water, and hydrogen fuel along with Polymer Electrolyte Membrane (PEM) fuel cell. It consists of three parts Solar panel, electrolyser and electricity production through fuel cell [4]. |
| Solar Panel: fuel prices constantly rising and global oil reserves on the verge of declining, renewable energy is what is being looked at, as never ending energy source of the future. Solar energy is most sought today in developing countries [5]. Conventional solar cells are called photovoltaic cells which are used to obtain solar energy. It identifies the direct conversion of sunlight into energy by means of solar cells. Photovoltaic refer to the creation of the voltage form light. Solar photovoltaic system directly converts sunlight into useful electricity. This process is called photoelectric effect, discovered by ‘Alexander Bequerel’ in 1839.The photovoltaic effect describes the release of positive and negative charge carriers in a solid state when light strikes its surface. The energy generator in a PV system is the solar cell. These solar cells are made out of semiconducting material, usually silicon. When light hits the cells, they absorb energy through photons. This absorbed energy knocks out electrons in the silicon, allowing them to flow. By adding different impurities to the silicon such as phosphorus and boron, an electric field can be established. This electric field acts a as a diode, because it only allow electrons to flow in one direction. Consequently, the end result is a current of electrons, better known to us as electricity. For given solar irradiation, the efficiency of PEC (Photo Electrochemical Cell) hydrogen energetic output is lower than the Photo Voltaic electrical output [6]. The output power (product of current and voltage) of a solar cell is temperature dependent. Higher cell temperature lead to lower output and hence to lower efficiency. The level of efficiency indicates how much of the radiated quantity of light is converted into usable electrical energy. The electrical system powered by solar arrays requires special design considerations due to varying nature of the solar power generated resulting from unpredictable and sudden changes in weather conditions which change the solar irradiation level as well as the cell operating temperature [7]. |
| Electrolysis Process: Electrolysis is a method of separating bonded elements and compounds by passing an electric current through them or it is defined as the decomposition of as substance by means of an electric current. When an electric current is passed through water containing an electrolyte, the water molecules decompose via an oxidationreduction reaction. Oxygen gas is generated at the anode and hydrogen gas is generated at the cathode as shown in Figure 4. |
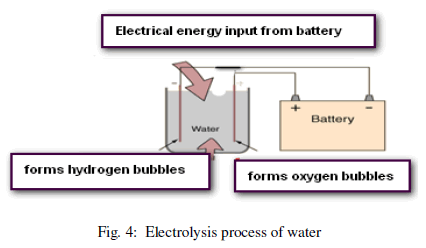 |
| The purpose of the electrolyte, such as sodium sulphate, is to provide ions that will carry the current through the solution. Depending on the nature of the electrolyte, different reactions may take place at the anode and the cathode during the electrolysis of an aqueous solution. |
| Hydrogen fuel cell: Hydrogen is an energy carrier that can be used to store, move, and deliver energy produced from other sources. It is the simplest element on the earth and the most abundant element in the universe; but despite its simplicity and abundance, hydrogen doesn’t occur naturally as a gas on earth. It is always combined with other elements for example water is a combination of hydrogen and oxygen. Hydrogen is also found in many organic compounds, notably the “hydrocarbons” that make up fuels such as gasoline, natural gas, methanol and propane etc. To generate electricity using pure hydrogen must first be extracted from a hydrogen containing compounds then it can be used in a fuel cell [8]. For example, in the electrolysis of water, electricity is used to produce hydrogen & oxygen. In a fuel cell the reverse reaction occurs; hydrogen and oxygen react to form water and electricity is produced. Whenhydrogen is burned, there is a reaction between the hydrogen and oxygen which results in water, while energy is release in the form of heat. In A fuel cell, the process is split in two parts. The two processes take place on each respective side of the electrolyte which keeps the gases separated, but which transports ions. The negatively charged electrons move in an outer electrical circuit. With this apparatus a portion of chemical energy is converted directly to electrical energy. In reality, the efficiency is lower, but compared to traditional technology, the fuel cell is very efficient. There are several fuel cells with different characteristics and uses. They are usually classified according to the electrolyte that is used i.e. alkaline (AFC), proton exchange membrane (PEMEC), phosphoric acid (PAFC), molten carbonate (MCFC), solid oxide (SOFC) etc. [2]. Polymer electrolyte membrane (PEM) fuel cell is also sometimes referred to as a polymer electrolyte fuel cell (PEFC). PEM fuel cells are the serious candidate for small-scale distributed stationary power generation [9]. |
METHODOLOGY |
| Investigations were carried out to study the temporal power analysis of solar-hydrogen based fuel cell system. In this research paper, the aim is to generate electricity using hydrogen fuel cell. The experiment setup can be understood below in figure 5. First of all, solar panel used solar energy and converted that solar energy into electrical energy. After conversion, solar panel applied that generated electrical energy to the electrolyser where electrolysis process of water takes place. When an electric current is passed through water containing an electrolyte, the water molecules decompose via an oxidation-reduction reaction. Oxygen gas is generated at the anode and hydrogen gas is generated at cathode, where oxygen and hydrogen gases are collected in different flasks. Now, collected pure hydrogen and the oxygen (in the form of air) are applied to a fuel cell. A fuel cell is an electrochemical device that converts hydrogen fuel directly into electricity and heat without combustion. By the nature of its electrochemical reaction, a fuel cell can be more than twice as efficient as an internal combustion engine. |
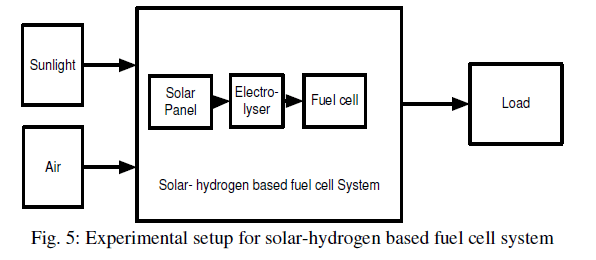 |
| A combustion engine burns fuel to create heat and in turn converts heat into mechanical energy and finally electricity. A fuel cell produces electricity, water and heat directly from hydrogen and oxygen and then applied generated renewable energy to the respective load. The hardware system of this experiment is shown in figure 6. In this experiment, solar module exposed to solar radiation from one hour with 460 ohm resistive load is connected with circuit. Then all readings are to be taken after one minute interval. |
 |
RESULTS AND DISCUSSION |
| Investigations were carried out using fuel cell system for the determination of the electrical properties of the solarhydrogen based fuel cell. It is discovered that power output reaches a maximum power point of 36.025 nW (nano- Watts) at 1.31 V (volts) and 27.5 μA (micro-Amperes) current. As the result, the maximum power output decreases to 1.029 nW (nano-Watts) at 0.21 V (volts) and 4.9 μA (micro-Amperes) from maximum power point of 36.025 nW (nano-Watts) at 1.31 V (volts) and 27.5 μA (micro-Amperes) current. The maximum power output of solar-hydrogen fuel cell setup taken during readings after solar panel was removed from the circuit. All readings are to be taken after every 1 minute as shown below in table 1. |
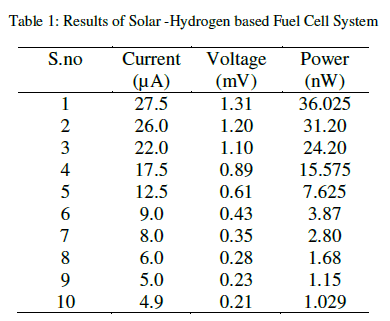 |
| The calculated values are represented in form of graph as shown below in figure 7. All of these are necessary in the concept of progress integration and cogeneration to reduce high energy consumption and difficulties within each unit. Solar-hydrogen pre-treatment section which is an essential part has been focus in this paper. Membrane technology is a promising alternative to be applied in this section and the overcome of PEM system performance after using this technology is in good agreement with primary mathematical simulation and the criterion of no external energy demand condition. |
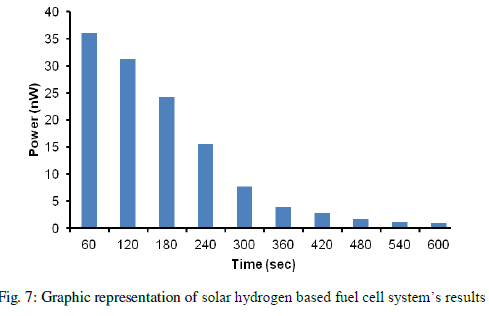 |
CONCLUSION |
| It can be concluded from the experimental study of solar-hydrogen based fuel cell system, the power of the system decrease with decrease the volume of hydrogen. It can provide appreciable stable voltage over time. Also, the waste products from system such as oxygen, heat and water are certainly not harmful for environment. As a result, we studied that however the volume of hydrogen decreases during electrolysis process, the output power will also decreases. So, this drawback can overcome by using hydrogen storage process during solar electrolysis process. |
ACKNOWLEDGMENT |
| The authors would like to express a deep sense of gratitude and thanks to Mr. Jang Bahadur Singh, Ph.D. scholar, Physics and Electronics, for extending his knowledge and help. His constant guidelines and encouragement made this research possible. |
References |
|