ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
FagbenroOluwakemi Kehinde1, Hamidi Abdul Aziz2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Sustainability of the environment has become a focus; hence the need for improvement and monitoring of textile waste water discharges, for which major concern is color. Color in textile dyeing and finishing effluents is as a result of large amounts of dye stuffs left during the dyeing stages and inefficient dyeing processes often resulting in unfixed forms. Waste water from Textile and dyeing industries contain many pollutants, making it high in organic and inorganic chemical content, total organic carbon (TOC), chemical oxygen demand (COD), and especially, strong color. It is thus difficult to be degraded by the conventional methods; hence the use of the advanced oxidation processes (AOPs). The advanced oxidative process is certainly not a very new treatment method as it has been studied and used extensively; however, hope remains for possible improvement, hence this review. AOPs are being researched more with a view to improve on the treatability of effluents and the hope to use the method for the complete mineralization of dyes. Although they are very effective in complete mineralization of pollutants, AOPs may be very expensive when used solely.
KEYWORDS |
| AOPs, Textile waste water, H2O2, UV, Color. |
1. INTRODUCTION |
| The importance of Textile industries in the manufacture of clothes and clothing materials cannot be over emphasized. But as good as they are, their existence also signifies a very crucial environmental issue, bothering on waste water discharges. According to [1], the textile industry is very important because its materials are used in varying ways including clothes for wearing. Products are however affected by the styles in clothing wears, changes with the season and the trends in fashion. |
| Dyes and colors are known to have a long history and constitute an important component in our daily lives. Natural plants and insect sources were initially used by the dye industry and then rapidly turned to synthetic manufacturing processes. The Synthetic dyes are considered a major part of our lives.[2, 3].Several of the synthetic dyes, especially azo dyes, were found to be toxic, carcinogenic and mutagenic and are thus banned throughout the world, [4, 5]. Their use and manufacture have however continued until today because of their low cost, ease of synthesis and other desirable properties [4, 6].Azo dyes are considered the largest group of dyes and or industrial colorants which currently represents 60-70% share in the worlds dye market [7-9]. The bonds are resistant to breakdown, hence, they exhibit the potential for persistence and accumulation in the environment, [4]. |
| The textile industries extensively use synthetic dyes in the dyeing and printing process, [4]generating a very large quantity of complex chemical substances which remained as unused materials in the wastewater from various stages of textile processing[3, 10-12]. |
| Also, the use of dyes by the textile industry has however grown steadily because they react well with fibers and their color is stable. Literatures have discussed dyes more, because of their high solubility in water and as effluents containing environmentally problematic compounds [13] in [14] that are visible in small quantities due to their brilliance,[15] |
 |
| The dyeing process in the textile processing generates much concern,as it uses an average of ten times more waterfor the preparation, dye washing, and rinsing stages [16]. Table 1.0 shows a list of most commonly used processes involved in textile fabric productions. |
| Textile dyeing and finishing generates the largest quantity of waste water as water is used for the cleaning of raw material and in many flushing stages in the wet production [17, 18].10-15% dyes are lost in effluent during the dyeing process [4]. The traditional textile finishing industry consumes about 100 liters of water to process about 1 Kg of textile material while the new closed-loop technologies such as the reuse of microbial or enzymatic treatment of dyeing effluents could help in reducing this enormous water pollution[4]. |
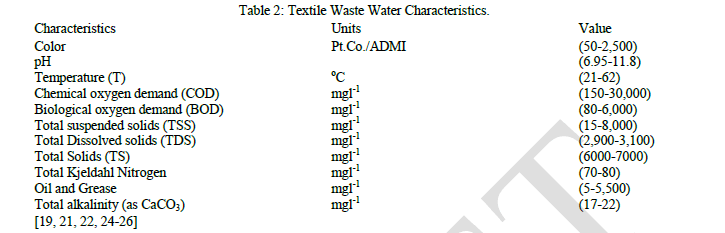 |
| Essentially, pollution due to effluent from textile industry has increased during the recent years and has become a great concern to a healthy environment. These waste waters are generally characterized by high content of dyestuff, salts, Biological Oxidation Demand (BOD),Chemical Oxygen Demand (COD), suspended solid (SS), heat, color, fluctuating pH, and the presence of metal ions, [4, 19]. Typical textile waste water characteristics are shown in Table 2.0. This review focuses on the progress and limitation of the advanced oxidative processes involving only Ultra Violet (UV) light and Hydrogen peroxide (H2O2) in the treatment of textile waste water in particular. |
| This review however, focuses on the progress of Ultra Violet (UV) light and Hydrogen peroxide (H2O2), in the advanced oxidative processes, for the treatment of textile waste water in particular. |
II.TREATMENT PROCESSES |
| There exists various techniquesfor the treatment of industrial effluents including textile waste water which are broadly categorized into physical, chemical and biological methods [27], or a combination of the methods such as the physicochemical and electrochemical methods. |
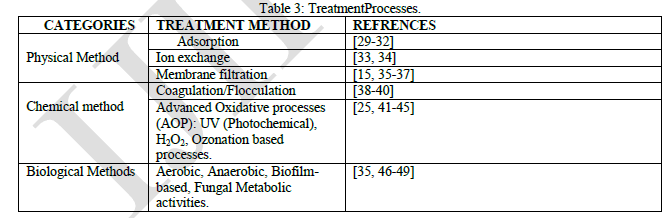 |
| Although Table 3 highlights the three basic category of treatment methods, a number of treatment technologies evolve thereof and are so much interrelated that a technique may be a part of two methods. Typically, adsorption can be physical or chemical, depending on the existing bonds formed in the process. |
III. ADVANCED OXIDATION PROCESSES (AOPS). |
| The advanced oxidative process is certainly not a very new treatment method as it has been studied extensively, but of course there is yet room for development of the process and the need to attain higher efficiencies,[50]. AOPs are generally considered as the set of chemical processes for the treatment of water and waste water by the reactions with hydroxyl radical (OH.) in oxidation. The AOPs are processes based on the generation and utilization of reactive species such as hydroxyl radicals (OH.), which is one of the strongest inorganic oxidants (E0=8V). The hydroxyl radicals oxidize a broad range of organic pollutants rapidly and non- selectively, [5, 51-53]. Hydroxyl radicals are very unstable and highly reactive because of their oxidation potential, [18]. |
| AOPs include also, both photocatalytic (involving Ultra violet light or Ultra Sound) and non-photocatalytic (dark) processes. AOPs emerge as an important destructive method for eliminating most of the organic and inorganic pollutants including reactive dyes. They have been used to enhance the biotreatability of wastewaters containing various organic compounds that are non-biodegradable and/or toxic to common microorganisms, hence AOPs in this sense are pretreatment methods, (Josmaria,L. et.al.2012)[3, 54, 55]. |
| More commonly, and in a more practical sense though, AOP refers to the treatment processes involving UV light, Ultrasound, Ozone (O3) and hydrogen peroxide (H2O2) either singly, serially or in combination with or without catalysts, such as metal ions or semiconductors,[53, 56], such as in the equations below . |
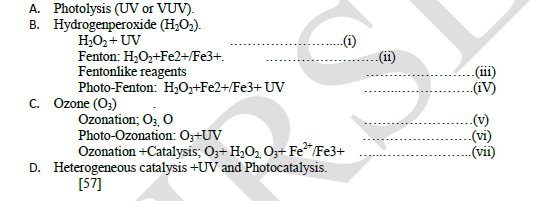 |
| The advanced oxidative processes (AOPs) are being researched more with a view to improve on the treatability of effluents and the hope to use the method for the complete mineralization of pollutants such as dyes to CO2, water, and inorganic compounds. However, AOPs will be very expensive when used solely, [10, 17,58]. |
| Because of the aforementioned problematic nature of dyeing effluents and due to the varying composition of the effluent, [28], also, as dye effluents are hardly biodegradable and are not very responsive to conventional physical and chemical treatment methods in singleton, and resulting in a simple transfer of pollutant from one phase to another rather than destroying them, which consequently leads to secondary pollution (Dongfang, Z. and Feng Z. 2012), therefore, the need arises for an effective treatment method for these waste water. |
| AOPs are therefore expected to be carefully monitored during operation, to avoid a partial oxidation of organic contaminants which may result in the formation of intermediates that are more toxic than the parent compounds as with the conventional treatment methods, [54, 58].3.1 Ultraviolet (UV) |
| 3.1 Ultraviolet (UV) |
| Ultraviolet (UV) radiation is considered as an essential element in photochemical oxidation for which the emitted wavelength plays a significant role. Such AOPs are mostly used to degrade compounds that absorb UV radiation within the corresponding range of the spectrum,[58]. |
| Ultraviolet (UV) have shown positive results for disinfection and removal of pathogens like estrogen 17α- ethinylestradiol (EE2), [59]. The effectiveness of ultraviolet (UV) reacting singly or in combination with other item/method can be dependent on several factors such as pH and initial concentration, [60]. |
| UV is sometimes enhanced in an AOP created by the addition of H2O2 to UV to destroy certain pathogens, and organic pollutants in water, [61]. |
| Also, the UV/H2O2-based processes have shown better performance with removal efficiencies higher than 80% for all investigated parameters obtained when it was integrated with the biological treatment, thereby meeting the discharge limits while no- biodegradability enhancement was shown when the process was used as an end treatment, [62]. |
| 3.2 Hydrogen Peroxide (H2O2). |
| H2O2 , can be used in various applications because of the different ways in which it functions selectively, and because it has no gaseous release nor chemical residues as found with other chemical oxidants, [53]. |
| However, negligible effects were observed in the presence of H2O2 alone, compared to the Fenton-mediated decoloration, [63]. Also, H2O2 alone (i.e., without UV power) proved ineffective as for both mineralizing and biodegradability enhancing agent and that the only reactive species was essentially the hydroxyl free radical OH., [62]. |
| The effect of operating conditions was observed when measuring via a spectrophotometer at the visible maximum absorption, it was found that the rate of decolorization increased with the initial dosage of H2O2 up to a maximum and beyond which decoloration was inhibited, [64]. |
| 3.3 UV/ H2O2, Processes. |
| The formation of the hydroxyl radical in the UV/ H2O2 is based on the photo dissociation of H2O2 by UV radiation in the range of 200-280nm,[50, 58]. |
| In the H2O2/UV process, the pH has shown to be a significant factor in dye discoloration and the process is also more effective in acidic media,[19]. |
| In a related treatment of textile dyeing wastewater, all the processes tested, using solar advanced oxidation processes contributed to an effective decolorization and mineralization, but the most efficient process was the solar-photo- Fenton[45, 65]. The first case had an optimum catalyst concentration of 60mg Fe2+L−1, leading to 98.5 % decolorization and 85.5 % mineraliza-tion after less than 0.1 and 5.8 kJ UVL−1, respectively. A final wastewater with a COD below 250 mg O2L−1 was achieved using the combination of a solar-photo-Fenton reaction with a biological process, which required a photo-treatment of 0.5 kJUVL−1,consuming 7.5 mM H2O2, resulting in 58.4 % of mineralization. The second case; using H2O2, with an optimum catalyst concentration of 100 mg Fe2+L-1 leading to 98% decolorisation and 89% mineralization after 7.2 and 49.1 kJUVL-1, respectively. |
| In an experiment [66],where the effect of ultrasound(US) and UV energies introduced into the Fenton like system were compared, 3 h after reaction, the removal rates of RB5, EDTA for, Total Organic Carbon(TOC), and Chemical Oxygen Demand (COD) were 100%, 96.5%, 68.6% and 92.2%, respectively. US showed a significant synergistic effect on the degradation and mineralization of both RB5 and EDTA, while UV did not achieve any improvement. |
IV.CONCLUSION |
| UV has shown positive results for disinfection and removal of pathogens and organic pollutants in water and waste water. The effectiveness of UV reacting singly or in combination with other methods is found to be dependent on factors which include especially, pH and initial concentration. |
| The UV/H2O2-based processes have shown better performance with removal efficiencies higher than 80% for the investigated parameters obtained when it was integrated with the biological treatment, thereby meeting the discharge limits while no- biodegradability enhancement was shown when the process was used as an end treatment. |
| The formation of the hydroxyl radical in the UV/ H2O2 process is based on the photo dissociation of H2O2 by UV radiation in the range of 200-280nm and H2O2 is variously applied because it has no gaseous release or chemical residues as found with other chemical oxidants. |
References |
|