e-ISSN: 2319-9849
e-ISSN: 2319-9849
Suresh Purohit and N Bhojak*
Green Chemistry Research Centre. PG Department of Chemistry, Govt. Dungar College (A-Grade), University of Bikaner, Bikaner – 334003, Rajasthan, India
Received: 18/02/2013; Revised: 13/03/2013; Accepted: 15/04/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Complexes of some lanthanides such as Pr (Praseodymium), Nd (Neodymium), Sm (Samarium) and Tb (Terbium) gives absorption bands in the visible region of the spectrum. The Pr(III), Nd(III), Sm(III) and Tb(III) ions have four, ten, seven and two peaks respectively in their absorption bands. On the other hand Gd (Gadolinium) gives absorption band in UV- region and it has two peaks in its absorption band.
spectra, lanthanide (III), absorption.
Lanthanide (III) ion is a subject of increasing interest in bioinorganic, biomedical, pharmaceutical and coordination chemistry. The coordination chemistry of lanthanide (III) ions with drugs having amide group, has been widely investigated over last two decades because of their medicinal and biochemical applications. They used as diagnostic tools in biomedical analysis as MRI contrast agents [1,2]. Lanthanide complexes have been found to exhibit anticancer and fungicidal properties [3].
The 4f-electrons of lanthanides yield two types of transitions such as f-f and f-d transitions. The f-f transitions which give rise to sharp, narrow bands of comparatively weak intensities which are Laporte forbidden, whereas allowed f-d transitions are relatively broad and intense. The observed spectral transitions of the lanthanide ions are f-f transitions. Since the 4f-sub-shell of Ln(III) ions is well shielded by the filled 5s and 5p sub-shells, the energy levels of the 4f-electrons are only little influenced by the environment of Ln(III) ion. The intensity of f-f transition is weak, because these transitions are Laporte forbidden. Relaxation of this selection rule is very less effective than that of d-d transitions. This is because of the weak crystal field interaction.
The Ln(III) [Pr(III), Nd(III), Sm(III), Tb(III) and Gd(III)] perchlorate were prepared by heating their oxides with perchloric acid and evaporating off the excess perchloric acid [4]. All glassware’s were first washed with chromic acid solution, thoroughly washed with water, rinsed with distilled water, ethanol, acetone, air dried and finally stored in a desiccators. The Ln(III) perchlorates and ligands (Acetaminophen and Indomethacin ) were dissolved separately in ethanol and then mixed. The reaction mixture was refluxed with constant stirring for 2h and the solution was concentrated to a viscous mass which was washed many times with distilled water and then ethanol. Finally this viscous mass dried over P4O10 in desicator.
The absorption bands of Pr(III), Nd(III), Sm(III), Tb(III) and Gd(III) complexes in the UV- visible and near IR region appear due to transitions from the ground levels 30H4, 4I9/2, 6H5/2, 7F6 and 8S7/2 respectively to the excited J-levels of 4fn-configurations. In all these cases nephelauxetic effect or red shift is observed. In all complexes increase in the intensity of the bands have been observed. The small values of bonding parameter (b1/2) observed in lanthanide complexes as compared to transition metals, suggest that the very less involvement of 4f-orbitals [5]. The ligands which show higher values of b1/2 in comparison to other ligands have more covalent than the latter ones and stronger ligands in a nephelauxetic sense [6-17]. Some important data of hypersensitive transitions have been given in tables. For Pr(III), Nd(III), Nd(III), Tb(III) and Gd(III) the hypersensitive transitions are –
For Pr(III)................ 3H4→3P2
For Nd(III)............... 4I9/2→4G5/2
For Sm(III)............... 6H5/2→6P3/2
For Tb(III)................ 7F6→5D3
For Gd(III)............... 8S7/2 →6D5/2
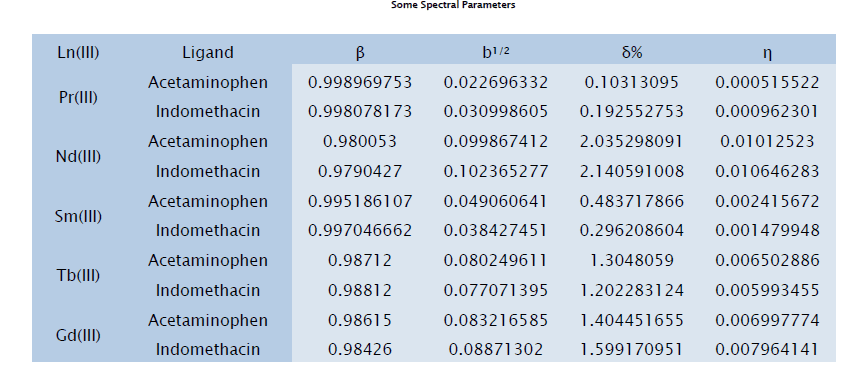
Some complexes of five Ln(III) ions have been prepared with ligands such as Acetaminophen and Indomethacin in ethanolic medium. Solution spectra of each complex have been recorded in UV-Visible and near IR range. By the help of some spectral data, some conclusions have made. The values of various parameters such as nephelauxetic ratio (β), bonding parameter (b1/2), Sinha’s covalency parameter (δ%) and Covalency angular overlap parameter (η) reveals that the involvement of 4f-orbital has negligible for lanthanide complexes as compared to transition metals.