ISSN: 2347-7830
ISSN: 2347-7830
Rajesh Kumar Singh*
Department of Chemistry, Jagdam College, JP University, Chapra, India
Received date: 12 December 2013 Accepted date:30 December 2013
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Butterflies are beautiful creation of nature. These species survival become very critical due to enhancement of corrosive pollutants in atmosphere. Corrosive pollutants react with moist oxygen and water to form inorganic and organic acids. The oxides of Carbon, oxides of nitrogen, oxides of sulphur, oxides of haloens and hydride of sulphur form carbonic acid, nitric acid, nitrous acid, sulphuric acid, sulphrous acid and halo acids. Organic acids are formic acid, acetic acid and benzoic acid. Butterflies come in contact of these corrosive acids to develop microbioelectrochemical corrosion cell. Oxidation and reduction reactions start on the body butterfly. It disturbs catabolic and anabolic process of butterflies. Such type of corrosion cell reaction destroys the life of butterflies. These corrosive pollutants reduce their population growth. The green house gases, acid rain, oxygen depletion and global warming are also producing bad effect their life. The above mention acids convert into form of cloud and they come on the earth surface as water droplets. The acid rain accelerates corrosion reaction with butterflies. The concentration of carbon dioxide and methane gages are increased in atmosphere day by day due to industrialization, transportation, urbanization, deforestation, burning of coal and infrastructure development work. These gases are increasing surrounding temperature of the earth in this way their life becomes critical. The corrosive gases, acid rain, oxygen depletion and green house gases become threat of their survival. The particulates are easily adhered with butterflies and they react with moisture to produce acid and base. It is major cause of corrosion with their body parts.
Butterfly, corrosive pollutants, microbioelectrochemical corrosion cell, galvanic, fertilization.
Our environment is polluted by pollutants, effluents, flue gases, human waste, and biological waste. animal waste, agricultural waste and municipal waste [1,2,3]. These sources are evolved corrosive and global warming gases into atmosphere [4,5]. Fulfillments of our basis need people are utilized natural sources unplanned ways, it another factors of increasing pollutants in environment. The other sources of corrosive pollutants are burning of coal, petroleum refinery, thermal power, sugar industry, pulp and paper industry, paint and dyes industry, beverage industry, food processing industry, pharmaceutical industry, slaughter house, chemical industry, chemical and biological laboratory, ceramic industry, glass industry, bakery industry, bricks formation industry, they can release harmful gases in nature [6,7].
Particulates come into atmosphere due to infrastructure development like industrialization, housing, hospital, road construction, rail tracks, telecommunications etc. These particulates are acidic and basic character. They react with moist air and water to from inorganic acids and bases [8,9].
Farmers use pesticide, insecticide and herbicide for the protection of crops. These chemicals are noxious for living organism [10,11]. Natural sources of pollutants [12] are electrical stomps, volcanic eruptions, and leakage of natural gas, photochemical reaction, respiration, soil and vegetation.
Man made sources of pollutants are transport [13], combustion of fuel, research laboratory, solid waste disposal, pesticide spray, deforestation, urbanization, construction work, roads, railways, water supply, cables for telecommunications, sewers, rocket lunching centre, nuclear explosion centre, household substances [14] and several other kinds of infrastructure.
Pollutant, effluent and hazardous waste create green house effect, acid rain, corrosiveness, pollute air and water, soil infertility, depletion in ozone layer, change in weather and health disorder. The purpose of this work is protection of materials, assets, health and environment by harmful effect of pollution. Spreading global awareness in both developed and developing countries public and industrialists to minimize every kind of release of pollutant, effluent and hazardous waste.
The equipment used for this work was gas analyzer and pH meter. Analysis of the concentration of corrosive pollutants like CO, CO2, SO2, SO3, NO2, H2S, and Cl- ion in different weathers. These corrosive gases were reacted with moisture to from H2CO3, H2SO3, H2SO4, HNO2, HNO3 and HCl. The pH values of these gases were measured by pH meter.
The corrosive pollutants enter in the atmosphere above mention sources. The oxides of carbon react with wet air to produce carbonic acid which exhibits H+ and bicarbonate ion. This acid develops bioelectrochemical corrosion cell with interaction with butterflies then oxidation and reduction reaction start on butterfly outer surface and corrodes its important organs. The bioelectrochemical corrosion reaction is mention in figure 1.
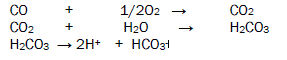
The oxides of sulphur absorb moist oxygen to form Sulphrous acid and sulphuric acid after dissociation of these acids generate H+ ions, sulphite ions and sulphates ion. These acids adhere with butterfly body and develop bioelectrochemical corrosion cell then after oxidation and reduction process occur on the surface of butterfly. Oxidation and reduction reactions are given in figure 2.
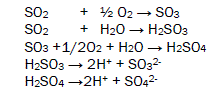
The oxides of nitrogen under go with wet atmospheric oxygen to form nitrous acid and nitric acid. They dissociate to produce H+ ions, nitrous ions and nitrates ions. Butterfly comes in surrounding these acids to form microbioeletrochemical cell and redox reaction occurs with this living species in this processes its several body parts corrodes. The microbioelectrochemical cell reaction is expressed in figure 3.
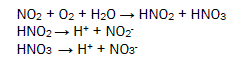
Chlorides ion absorbs moisture to produce hydrochloric acid and hypochlorous acid. Hydrogen sulphide forms sulphur dioxide to react with dry oxygen and it absorbs wet oxygen to give sulphuric acid. Formic acid, acetic acid and benzoic acid produce H+ ions which creates microbiological cell with butterfly then accelerate corrosion reaction. This reaction is destroying biological organs of butterfly. The biological reaction is mention in figure 4.
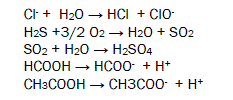
Corrosive pollutants are global problems in front of world community. Developed and developing countries release huge amount of above mentioned harmful substances in atmosphere. It creates new problems like abrupt change in weather, global warming, acid rain, human disease, makes land infertile, decrease food grain production corroding living and non-living species. It is observed that living and non-living organism are badly affected in industrial and urban area because these pollutants are found more in this area. National and International pillars, bridge, building, railways, museum, sculpture, pipe, metallic equipments, bimetallic metals are facing severe corrosion problems. This is a vital issue in front of world community to save our national and international heritage from corrosion. To check corrosion of material the government and private organizations expense huge money and it is major setback for our economy. Poor people of developing counties are highly affected by corrosive pollutants.