ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Satya Prakash Saxena1, Sunita Chawla2, Shilpa Gupta3, Nikhil Rastogi4 and Manish Saxena3*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The compositional effect on some physical properties with the variation in bismuth content has been studied theoretically for Ge-Se-Bi glassy semiconductors. The present Ge-Se-Bi system viz: Ge10Se90-xBix, Ge14Se86-xBix, Ge18Se82-xBix (x = 4, 8, 12, 16, 20, 24 at. %) is found to be in accordance with the earlier researches according to which system with large number of lone-pair electrons constitutes a stable state. The addition of Bi to Ge-Se glassy alloys supposed to change in the whole category of physical properties. It has been found that almost all the parameters, studied here, vary linearly with the increase in Bi content, thus making this suitable for phase change optical recording. The phase change can be reversibly switched between the amorphous and crystalline state and find applications in rewritable optical recording.
Keywords |
| Chalcogenide Glasses, Coordination Number, Average Heat of Atomization, Cohesive Energy. |
INTRODUCTION |
| Study of chalcogenide glasses received so much attention know a days because of their unique properties and large applications. Chalcogenide glasses form an important class of amorphous solids. Chalcogenide glasses contain one or more of the chalcogen elements, S. Se or Te of the sixth group of the periodic table. They have very interesting physical properties and can be prepared in the bulk as well as thin film forms. One of the greatest advantages of these glasses is the composition dependent tunability of their properties, which enables one to design materials for specific requirements. They have potential technological applications such as materials for threshold and memory switching, inorganic photoresist, xerography, IR detection and transmission etc. [1,2]. There exist many kinds of amorphous chalcogenides, and classification may be valuable. The amorphous chalcogenide can be classified into the elemental, binary, ternary, etc., and the alloys can be divided into stoichiometric (As2S3, GeSe2) and non-stoichiometric compositions (S–Se, As–Se). For the elements, the sequence of S, Se, and Te shows that bonding changes from molecular, covalent, to metallic. Among these three elements, only Se is available as amorphous films and glassy ingots at room temperature. The glassy structure is the simplest, consisting mainly of entangled−Se−Se− chains. In addition, depending upon preparation procedures, small amounts of ring molecules such as Se8 may be included. Note that the stable structure of c-Se is composed of aligned helical chains. An important property of a-Se is that the material exhibits peculiar photoconducting properties, which have been and are widely applied to photoconductor devices. For ternary alloys, we can envisage several kinds of systems: the chalcogen mixture S–Se–Te, the anion-mixed system such as As–S–Se, and the cation-mixed system such as Ge–Sb–Te [3, 4]. |
| There is a lot of scope left even today, in the understanding of electronic states in the chalcogenides. The absence of rigidity of the structure in chalcogenides makes them suitable for various applications. Selenium is mostly used because of its wide commercial importance. The addition of Bi increases the chemical durability and broadens the IR transparency region. Earlier worker measured the optical gap and electrical resistivity of a series of Ge20BixSe80-x glasses with (x=0, 5, 10, 15, 20, 25) [5]. They analyzed the absorption spectra of films with various Bi compositions to determine the optical band gap. It was reported that the optical band gap decreased sharply from 2.10 eV to 1.65 eV with the increase in the Bi contents from 0 to 10 at. % . The optical and electrical results suggest that the addition of Bi produces localized states near the conduction – band edge, so the electrical transport is due to hopping of electrons after being excited into localized states at the conduction-band edge. |
| In the present work, we have selected Ge-Se-Bi glasses to study the compositional variation on physical properties. We have taken three combination viz. Ge10Se90-xBix, Ge14Se86-xBix, Ge18Se82-xBix (x = 4, 8, 12, 16, 20, 24 at. %) to study the physical properties, viz.: average coordination number, average heat of atomization, cohesive energy etc., by varying Bi content in all compositions as well as by increasing the Ge content from 10 to 18 and hence subsequently by decreasing selenium content in order to check the importance of Ge-Se-Bi glass system for optical recording devices. |
BONDING CONSTRAINTS & AVERAGE COORDINATION NUMBER |
| Two models namely, RCNM (Random Covalent Network Model) and CONM (Chemically Ordered Network Model) have been reported to be consistent with the bonding schemes (the 8-N rule) in chalcogenide network glasses [6, 7]. Out of these two, the CONM has been adopted to understand the features observed in the property-composition dependence of a very wide range of chalcogenide glasses [8, 9]. The CONM is also referred as the change crossing model or as the COCN (Chemically Ordered Covalent Network Model) [10]. The formation of hetropolar bonds is favoured over the formation of homopolar bonds in CONM. According to CONM the glass structure can be composed of cross linked structural units of GeSe2 and Bi2Se3 with excess of Ge or Se dispersed among Ge-Se-Bi glass system. Though the CONM was adopted successfully to understand the features observed in the property – composition dependence of several systems, later it was found that aspects such as the ease of glass formation and stability of specific composition were not addressed to in this model. With the advent of efficient preparation techniques, the glass formation regions of some systems could be extended. Out of these models, one of which was developed by Phillips and Thrope [11, 12], the dynamics of network topology has been given importance. The CONM can account for only one of the features observed in V-VI (P, As, Sb, S, Se, Te) and IV-V (Ge, Si, S, Se, Te) systems. The topological models on the other hand can account for features at both the Z values (Z ~2.40 and 2.67). It was, therefore, thought that the topological models are of a more general nature and therefore these models began to get wider acceptance in understanding the features observed in the property-composition dependence of chalcogenide network glasses. The ease of glass formation and the stability of the resulting network are also associated with the value of Z. Special features observed in the Z data in a large number of systems around the threshold value of Z have been attributed to this topological transition which has been probed by studying both macroscopic and microscopic properties [13, 14]. The average coordination number (Z) is calculated using standard method [15] for the three composition of Ge-Se-Bi system, Z is given by |
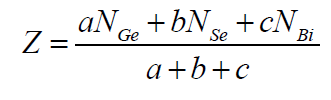 |
| where a, b and c are the at. % of Ge, Se and Bi respectively and NGe(4), NSe(2), NBi(3) are their respective coordination number [15,16]. From the calculated values of average coordination number for Ge10Se90-xBix, Ge14Se86-xBix, Ge18Se82- xBix (x = 4, 8, 12, 16, 20, 24 at. %) systems , it is clear from figure 1 that values of Z increase with increase in concentration of Bi from 4 to 24 as well as increase in Ge content from 10 to 18 in Ge-Se-Bi system. |
DEVIATION FROM THE STOICHIOMETRY OF COMPOSITION |
| The parameter R also plays an important role in the analysis of the results. Depending on R values, the chalcogenide systems can be organized into three different categories: |
| • For R = 1, the system reaches the stoichiometric composition since only hetero polar bonds are present. |
| • For R > 1, the system is chalcogen-rich. There are hetero-polar bonds and chalcogen–chalcogen bonds present. |
| • For R < 1, the system is chalcogen-poor. There are only hetero-polar bonds and metal–metal bonds present. |
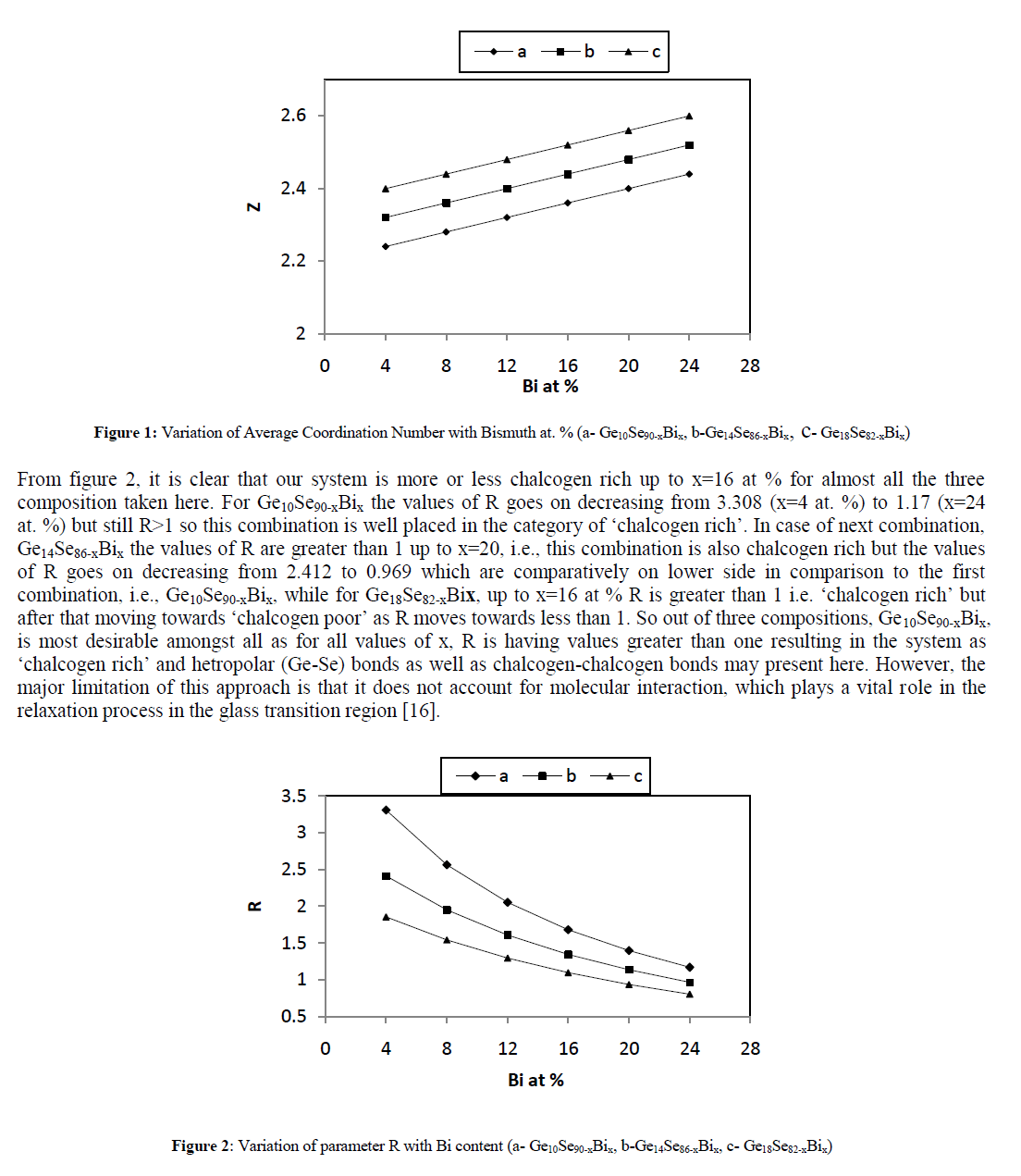 |
AVERAGE HEAT OF ATOMIZATION, COHESIVE ENERGY AND BAND GAP |
| As proposed by Pauling[17, 18], in case of ternary and higher order semiconductor materials, the average heat of atomization Hs is defined for a compound Aa Bb Cc is considered as a direct measure of the cohesive energy and thus average bond strength, as |
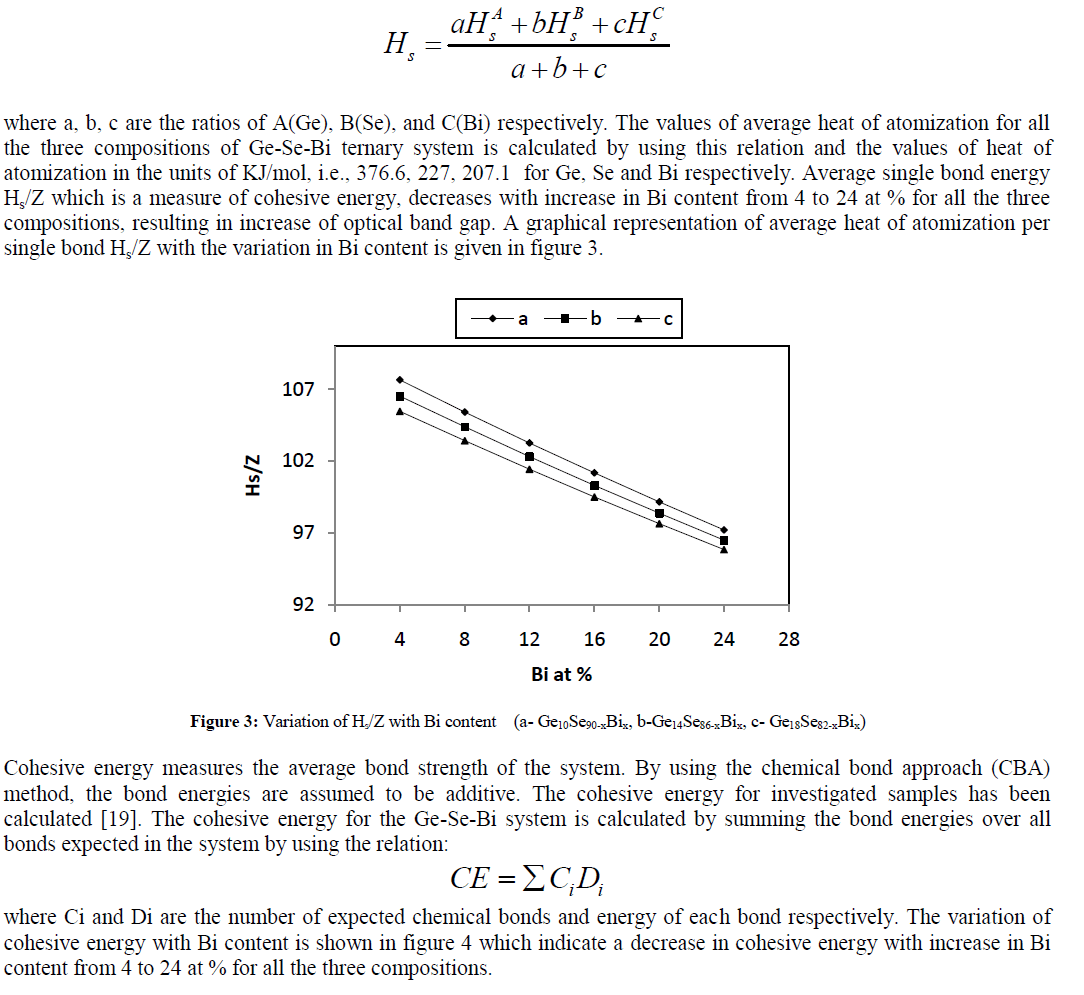 |
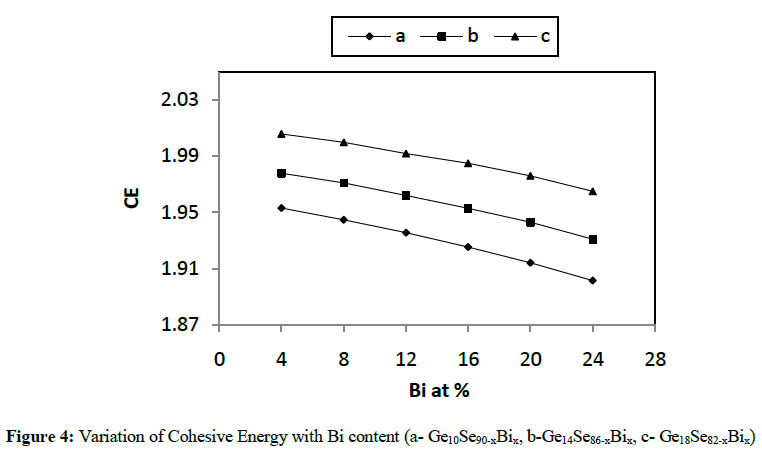 |
| It has been observed that the variation in the values of theoretical band gap (Eg) with composition can be described by the following relation [20]: |
| Eg(ABC) = aEg(A)+ bEg(B)+ cEg(C) |
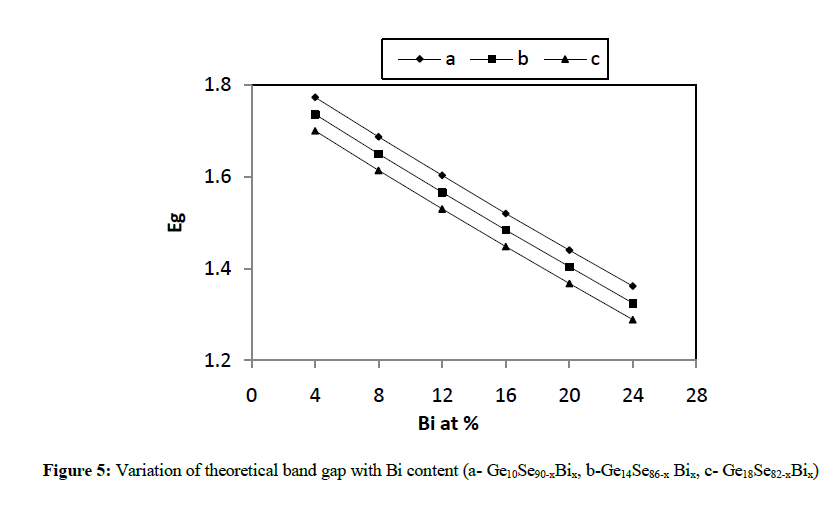
| where a, b, and c are the volume fractions and Eg(A), Eg(B) and Eg(C) are the optical gaps of elements A, B and C respectively. The values of theoretical band gap (Eg) decreases with the increase in the Bi content from 4 to 24 at % as depicted in figure 5. |
 |
CONCLUSION |
| In the present work, various physical parameters properties viz. average coordination number, average heat of atomization, cohesive energy etc., have been calculated theoretically for different combinations like Ge10Se90-xBix, Ge14Se86-xBix, Ge18Se82-xBix (x = 4, 8, 12, 16, 20, 24 at. %) of Ge-Se-Bi glassy alloys. It has been concluded from various figures given above that values of almost all the parameters vary linearly with increase in concentration of Bi from 4 to 24 at. % as well as increase in Ge content from 10 to 18 in Ge-Se-Bi system. The value of parameter R shows that our system is more or less chalcogen rich for all the three compositions. The results here clearly depict variations in average heat of atomization and cohesive energy with increasing the content of Bi from 4 to 24 for all the three compositions and hence confirming the status of above mentioned combinations good for optical storage media. |
References |
|